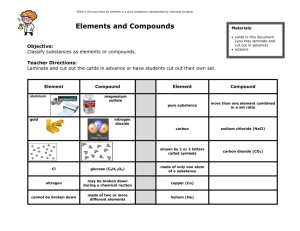
On level test
advertisement
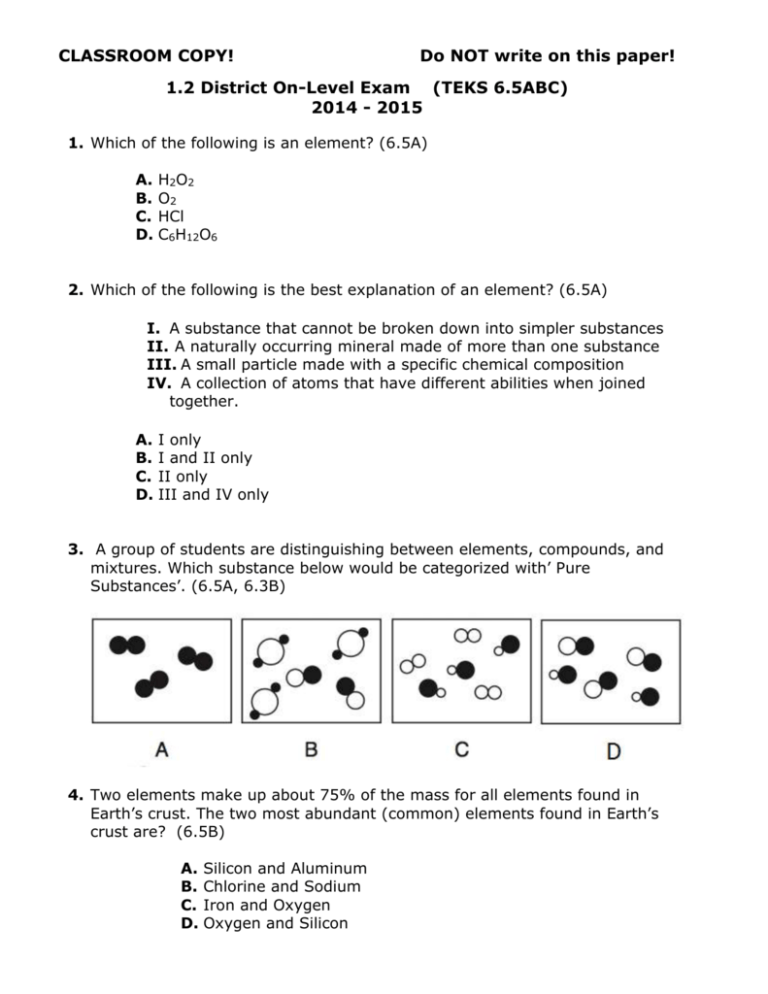
CLASSROOM COPY! Do NOT write on this paper! 1.2 District On-Level Exam (TEKS 6.5ABC) 2014 - 2015 1. Which of the following is an element? (6.5A) A. B. C. D. H2O2 O2 HCl C6H12O6 2. Which of the following is the best explanation of an element? (6.5A) I. A substance that cannot be broken down into simpler substances II. A naturally occurring mineral made of more than one substance III. A small particle made with a specific chemical composition IV. A collection of atoms that have different abilities when joined together. A. B. C. D. I only I and II only II only III and IV only 3. A group of students are distinguishing between elements, compounds, and mixtures. Which substance below would be categorized with’ Pure Substances’. (6.5A, 6.3B) 4. Two elements make up about 75% of the mass for all elements found in Earth’s crust. The two most abundant (common) elements found in Earth’s crust are? (6.5B) A. B. C. D. Silicon and Aluminum Chlorine and Sodium Iron and Oxygen Oxygen and Silicon 5. Which graph below best shows the composition of Earth’s atmosphere? (6.5B) A C B D 6. Which statement is the best comparison of elements and compounds? (6.5C) A. Elements are pure substances that contain only one type of atom, and compounds are mixtures of elements. B. Elements are two or more atoms chemically bonded together, and compounds are pure substances that contain only one type of atom. C. Elements are pure substances that contain only one type of atom, and compounds are two or more different atoms chemically bonded together. D. Elements are mixtures of pure substances, and compounds are mixtures of different substances. 7. The formula for Sodium Chloride (salt) is NaCl. Sodium Chloride is a(n) _________________. (6.5C) A. B. C. D. A A A A compound pure substance mixture solution 8. Use the information in the table below to answer the following question. Element Hg I Li Compound H2O NaCl C6H12O6 Based on the pattern in the table, is O2 a compound? (6.5C) A. B. C. D. Yes, because it contains two atoms. No, because it is not a pure substance. Yes, because it is not a pure substance. No, because it does not contain at least two different atoms. 9. A science student is assigned to write a paper on a common compound. Which substance could the student choose for this assignment? (6.5C) A. B. C. D. Hydrogen Gold Water Helium 10. Sulfur reacts with other elements to form new substances, but sulfur cannot be broken into simpler substances. Based on this information, how is sulfur categorized? (6.5A) A. B. C. D. Solution Element Compound Mixture 11. Part of the periodic Table is shown below. Read the answer choices carefully. Which statement most accurately classifies these substances? (6.5C) A. Hydrogen and potassium are elements because their symbols only contain one letter. B. Lithium, beryllium, sodium, magnesium, and calcium are elements because their symbols contain two or more letters. C. Hydrogen, lithium, beryllium, sodium, magnesium, potassium, and calcium are all elements, because only elements are listed on the Periodic Table. D. Hydrogen, lithium, beryllium, sodium, magnesium, potassium, and calcium are not elements because only compounds are listed on the Periodic Table. 12. Of the substances pictured below, which model represents a compound? (6.3B, 6.5C) A. C. B. D. 13. Which substance in the chemical reaction is a compound? (6.5C) Fe + S FeS A. Fe C. FeS B. S D. Not here 14. When magnesium (Mg) metal is burned in the presence of Oxygen (O2), magnesium oxide (MgO) is produced. The properties of magnesium oxide are different than the individual properties of magnesium and oxygen because magnesium oxide is ___________________. (6.5C) A. B. C. D. A solution A mixture A compound An element 15. Describe the product of the following chemical reaction. (6.5C) CaCO3 A. B. C. D. One One Two Two CaO + CO2 element compound elements compounds 16. Carbon dioxide (CO2) is not found on the Periodic Table because carbon dioxide is ________________. (6.5C) A. B. C. D. An atom A compound A gas A mixture 17. Look at the equation below. CH4 + 2O2 CO2 + 2 H2O How many different elements are in the reaction? (6.5C) Record your answer in the grid on your answer document. 18. The majority of the Earth’s oceans are composed of water (H2O). If the elements Hydrogen and Oxygen make up most of the Earth’s oceans, then what are the two elements that make up the salinity of the ocean? (6.5B) A. B. C. D. Nitrogen and sulfur Sodium and chloride Silicon and aluminum Fluorine and carbon 19. The chemical equation for hydrochloric acid is H + Cl HCl. Which correctly identifies each side of the chemical equation? (6.5C) A. B. C. D. elements compound compounds compound elements element compounds mixture 20. Look at the given compound. Be3Al2(SiO3)6 How many different elements are in the compound? (6.5C) Record your answer in the grid on your answer document.