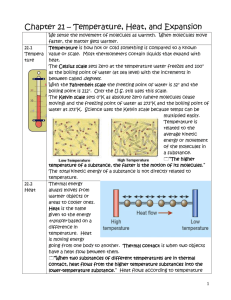
Heat is the thermal energy transferred from one
advertisement

THERMAL PHYSICS - SUMMARY Temperature (Definition #1 – Macroscopic level): a property that determines the direction of thermal energy transfer between two objects ▪ gives indication of the degree of hotness or coldness of a body, measured by thermometer Temperature (Definition #2 – Microscopic level): a measure of the average random kinetic energy of all the particles of a system ▪ (absolute) temperature is directly proportional to the average kinetic energy of the molecules of a substance: (KE) = 3⁄2 kT (k is Boltzmann constant) avg Kinetic energy of the molecules arises from random translational vibrational and rotational motion. Potential energy of the molecules arises from the intermolecular forces (bonds). Internal energy / Thermal energy is total potential energy and random kinetic energy of the molecules of a substance Heat is the thermal energy transferred from one body or system of higher temperature to another of lower temperature. Thermal equilibrium occurs when all parts of the system are at the same temperature. There is no thermal energy transfer. (This is how a thermometer works) Atomic mass unit is the mass of 1/12 of the mass of a carbon-12 atom. Relative atomic mass is the mass of an atom in units of 1/12 of the mass of a carbon-12 atom. The mole is the amount of substance that contains the same number of atoms/molecules as 0.012 kg of carbon-12. Molar Mass is the mass of one mole of a substance (kg/mol). 1 mole of a gas at STP occupies 22.4 l (dm3) and contains 6.02 x 1023 molecules/mol. Heat/Thermal Capacity is the amount of thermal energy needed to raise the temperature of a substance/object by one degree Kelvin. Q C = ∆T unit: (C) = J K-1 Amount of energy needed to raise temperature of an object by ∆T K is ∆Q = C∆T Specific heat capacity is the amount of thermal energy required to raise the temperature of one kilogram of a substance by one degree Kelvin. c = 𝑄 𝑚∆𝑇 unit: (c) = J kg-1 K-1 Amount of energy needed to raise temperature of 1 kg of a substance by ∆T K is homogeneous substance: C = mc ∆Q = cm∆T Thermal/heat capacity – object “specific” is ‘per kg’ of a substance Latent heat is the thermal energy that a substance/body absorbs or releases during a phase change at constant temperature. Qat const. temp. unit: J Specific latent heat is the thermal energy required for a unit mass of a substance to undergo a phase change. L= Q m unit: (L) = J/kg Amount of energy needed to change the phase of 1 kg of a substance is ∆Q = cm∆T If electrical energy is converted into increase of internal energy of the system, then: Qadded = electrical energy = Pt = IVt = Qabosorbed P – power, I – current, V – voltage, t - time 4 Phases (States) of Matter Characteristics solid, liquid, gas and plasma; ordinary matter – only three phases Solid Liquid Gas definite volume and definite shape definite volume but its shape can change – it takes the shape of their containers. neither definite volume nor definite shape Almost Incompressible Very Slightly Compressible Highly Compressible Bonds = intermolecular forces characterized by high density and the molecules are held in fixed position by strong bonds. Molecules vibrate around a mean (equilibrium) position. density is lower and molecules are further apart without fixed positions Molecules experience little resistance to motion and move freely about. There are still strong forces between the molecules but they are free to move around each other. the forces between molecules are very weak – molecules are essentially independent of one another but they do occasionally collide Comparative Density High High Low Kinetic Energy Vibrational Vibrational, rotational, some translational Mostly translational, higher rotational and vibrational Potential Energy High Higher Highest (ideal gas – zero) Volume and shape Compressibility Mean molecular Separation r0 ( size of the particle) > r0 10 - 100 r0 Phase transition is the transformation of a thermodynamic system from one phase to another. Changes: S → L melting or fusion S → G sublimation L → G vaporization includes boiling and evaporation L → S freezing or solidification G → S deposition or desublimation G → L condensation While melting, vibrational kinetic energy increases and particles gain enough thermal energy to break from fixed positions. Potential energy of system increases. Melting point of a solid is the temperature at which it changes state from solid to liquid. Once at the melting point, any additional heat supplied does not increase the temperature. Instead is used to overcome the forces between the solid molecules increasing potential energy. ◌ At the melting point the solid and liquid phase exist in equilibrium. While freezing, particles lose potential energy until thermal energy of the system is unable to support distance between particles and is overcome by the attraction force between them. Kinetic energy changes form from vibrational, rotational and part translational to merely vibrational. Potential energy decreases (It is negative!!! – attraction – intermolecular forces become stronger). While boiling, substance gains enough potential energy to break free from inter-particle forces. Similar to evaporation, the only difference being that energy is supplied from external source so there is no decrease in temperature. While condensing, the energy changes are opposite to that of boiling. The distinguishing characteristic of a phase transition is an abrupt change in one or more physical properties, in particular the heat capacity, and the strength of intermolecular forces. During a phase change, the thermal energy added or released is used to change (increase/decrease) the potential energy of the particles to either overcome or succumb to the inter-molecular force that pulls particles together. In the process, the average kinetic energy will not change, so temperature will not change. Evaporation is a change of phase from the liquid state to the gaseous state that occurs at a temperature below the boiling point. Evaporation causes cooling. A liquid at a particular temperature has a range of particle energies, so at any instant, a small fraction of the particles will have KE considerably greater than the average value. If these particles are near the surface of the liquid, they will have enough KE to overcome the attractive forces of the neighbouring particles and escape from the liquid as a gas. The escape of the higher-energy particles will lower the average kinetic energy and thus lower the temperature. The rate of evaporation is the number of molecules escaping the liquid per second. Evaporation can be increased by • increasing temperature/more particles have a higher KE • Increasing surface area/more particles closer to the surface • Increasing air flow above the surface (gives the particles somewhere to go to)/ decreasing the pressure of the air above liquid FAMOUS IB QUESTION: Distinguish between evaporation and boiling. Evaporation – process whereby liquid turns to gas, as explained above - occurs at any temperature below the boiling temperature - occurs only at surface of liquid as molecules escape - causes cooling of liquid Boiling – process whereby liquid turns to gas when the vapor pressure of the liquid equals the atmospheric pressure of its surroundings - occurs at one fixed temperature, dependent on substance and pressure - occurs throughout liquid as bubbles form, rise to surface and are released temperature of substance remains constant throughout process Kinetic Model of an Ideal Gas help: PV = NkT P – pressure, V – volume, N number of particles, k – Boltzmann constant, T - temperature Gas pressure is the force/area gas molecules exert due to their collisions (with a wall – imaginary or real). P= 𝐹 A (pressure = force/area) Assumptions of the kinetic model of an ideal gas • Gases consist of tiny hard spheres/particles called atoms or molecules. • The total number of molecules in any sample of a gas is extremely large. • The molecules are in constant random motion. • The range of the intermolecular forces is small compared to the average separation of the molecules. • The size of the particles is relatively small compared with the distance between them. • No forces act between particles except when they collide, and hence particles move in straight lines. • Between collisions the molecules obey Newton’s Laws of motion. • Collisions of short duration occur between molecules and the walls of the container and the collisions are perfectly elastic (no loss of kinetic energy). Macroscopic behavior of an ideal gas in terms of a molecular model. • Increase in temperature is equivalent of an increase in average kinetic energy (greater average speed). This leads to more collisions and collisions with greater impulse. Thus resulting in higher pressure. • Decrease in volume results in a smaller space for gas particles to move, and thus a greater frequency of collisions. This results in an increase in pressure. Also, depending on the speed at which the volume decreases, particles colliding with the moving container wall may bounce back at greater speeds. This would lead to an increase in average kinetic energy and thus an increase in temperature. • An increase in volume would have an opposite effect. Application of the "Kinetic Molecular Theory" to the Gas Laws Microscopic justification of the laws Pressure Law (Gay-Lussac’s Law) – Effect of a pressure increase at a constant volume Macroscopically: at constant volume the pressure of a gas is proportional to its temperature: V = NkT → P = (const) T Microscopically: ▪ As T increases, KE of molecules increase ▪ That implies greater change in momentum when they hit the wall of the container ▪ Thus microscopic force from each molecule on the wall will be greater ▪ As the molecules are moving faster on average they will hit the wall more often ▪ The total force will increase, therefore the pressure will increase The Charles’s law – Effect of a volume increase at a constant pressure Macroscopically: at constant pressure, volume of a gas is proportional to its temperature: PV = NkT → V = (const) T Microscopically: ▪ An increase in temperature means an increase in the average kinetic energy of the gas molecules, thus an increase in speed ▪ There will be more collisions per unit time, furthermore, the momentum of each collision increases (molecules strike the wall harder) ▪ Therefore, there would be an increase in pressure ▪ If we allow the volume to change to maintain constant pressure, the volume will increase with increasing temperature Boyle-Marriott’s Law – Effect of a pressure decrease at a constant temperature Macroscopically: at constant temperature the pressure of a gas is inversely proportional to its volume: PV = NkT → P = (const)/V Microscopically: ∎ Constant T means that the average KE of the gas molecules remains constant ∎ This means that the average speed of the molecules, v, remains unchanged ∎ If the average speed remains unchanged, but the volume increases, this means that there will be fewer collisions with the container walls over a given time ∎ Therefore, the pressure will decrease