Chapter_16
advertisement

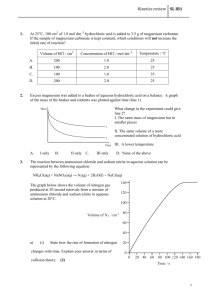
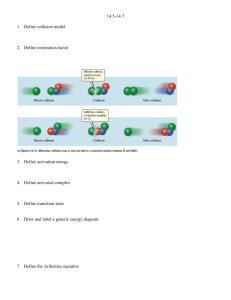
Multiple Choices 1. The change in concentration of a reactant or product per unit of time is called: (Objective 16.1.1) a. collision theory b. activated complex c. activation energy d. reaction rate 2. The minimum amount of energy that reacting particles must have to form the activated complex and lead to a reaction is called: (Objective 16.1.4) a. collision theory b. activated complex c. activation energy d. reaction rate 3. Adding a(an) ------------------- to a chemical reaction slows down its rate. (Objective 16.2.2) a. enzyme b. inhibitor c. energy d. catalyst 4. The substance that increases the rate of a chemical reaction without being consumed in the reaction is called: (Objective 16.2.2) a. catalyst b. inhibitor c. activated complex d. none of the above 5. Which of the following statements about a catalyst is true? (Objective 16.2.2) a. A catalyst can initiate a reaction. b. A catalyst can accelerate a reaction. c. A catalyst can be consumed during a reaction. d. A catalyst can be changed during a reaction. 6. Which of the following factors does NOT affect the rate of the reaction? (Objective 16.2.1) a. the amount of the reactants c. the size of the container used b. the physical state of the reactants d. temperature 7. A/An _____ lowers the activation energy required for a reaction to take place. (Objective 16.2.3) a. catalyst c. Reactant b. inhibitor d. product 8. A collision requires _____ to be effective. (Objective 16.1.3) a. only enough energy b. favorable orientation c. enough energy and favorable orientation d. a reaction mechanism 9. If a collision between molecules is very gentle, the molecules are: (Objective 16.1.3) a. more likely to be oriented favorably. b. less likely to be oriented favorably. c. likely to react. d. likely to rebound without reacting. 10. A rate law relates: (Objective 16.2.1) a. reaction rate and temperature. c. temperature and concentration. b. reaction rate and concentration. d. energy and concentration. 11. Hydrogen iodide (HI) decomposes as . The average reaction rate is expressed as: Average rate – . The negative sign used in the rate expression indicates that: (Objective 16.1.2) a. There are repulsive forces between the reactants. b. The concentration of HI decreases with time. c. The concentration of the reactants is less than that of the product. d. The reaction rate is decreasing with time. Problems 1. Use the data in the following table to calculate the average reaction rates for (N2) and (H2). (Objective 16.1.2) Experimental data for N2 + 3H2 2NH3 Time (s) [N2] (M) [H2] (M) [NH3] (M) 0.00 0.60 0.80 0.00 7.00 0.40 0.30 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛 𝑟𝑎𝑡𝑒 = − ∆ [𝑟𝑒𝑎𝑐𝑡𝑎𝑛𝑡] ∆𝑡 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛 𝑟𝑎𝑡𝑒 𝑜𝑓 (𝑁2 ) = − [𝑁2 ]𝑎𝑡 𝑡2 − [𝑁2 ]𝑎𝑡 𝑡1 0.40𝑀 − 0.60𝑀 − 0.20𝑀 = − = − 𝑡2 − 𝑡1 7.00 𝑠 − 0.00 𝑠 7.00 𝑠 = 0.0286 𝑀⁄𝑠 = 0.0286 𝑚𝑜𝑙 ⁄(𝐿. 𝑠) 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝑟𝑒𝑎𝑐𝑡𝑖𝑜𝑛 𝑟𝑎𝑡𝑒 𝑜𝑓 (𝐻2 ) = − [𝐻2 ]𝑎𝑡 𝑡2 − [𝐻2 ]𝑎𝑡 𝑡1 0.30𝑀 − 0.80𝑀 − 0.50𝑀 = − = − 𝑡2 − 𝑡1 7.00 𝑠 − 0.00 𝑠 7.00 𝑠 = 0.0714 𝑀⁄𝑠 = 0.0714 𝑚𝑜𝑙 ⁄(𝐿. 𝑠) 2. Use the following diagram to show the positions of each of these terms: reactants, products, activated complex, activation energy, and Δ H of the reaction. (Objectives 16.1.5) 1- Activation energy 2- Activated complex 3- Δ H of the reaction 4- Reactants 5- Products 3. Draw a graph to show how the use of a catalyst affects the energy released in the reaction. (Objective 16.2.3) 4. Octane on reaction with chlorine (Cl2) produces octyl chloride and hydrogen chloride (HCl). Calculate the final concentration of octyl chloride when the initial concentration of the product is 0.80 and the average reaction rate is 0.74 mol/(L s). The data of time is given below. (Objective 16.1.2) Time (s) Initial time 6.00 Final time 13.8 6.6 mol/(L s) 5. The reaction between sulfur and oxygen produces sulfur dioxide. The data is given in the table below: (Objective 16.1.2) Time (s) [S] (M) [O2] (M) [SO2] (M) t1 12 0.082 0.067 0.000 t2 18 0.040 0.020 0.041 a. Calculate the average reaction rate expressed in moles of sulfur consumed per liter per second. 0.0070 mol/(L s) b. Calculate the average reaction rate expressed in moles of oxygen consumed per liter per second. 0.0079 mol/(L s) c. Calculate the average reaction rate expressed in moles of sulfur dioxide consumed per liter per second. 0.0068 mol/(L s) 6. In a reaction of butyl chloride and water, the concentration of butyl chloride is 0.220M at the beginning of the reaction. After 4.00 seconds, the concentration of butyl chloride is 0.100M. Calculate the average reaction rate over the given time period expressed as moles of butyl chloride consumed per liter per second. (Objective 16.1.2) 0.0300 mol/ L s -(0.001M - 0.220M / 4 s - 0 s) Short Answers 1. Explain why collisions between two reacting particles do not always yield a product. (Objective 16.1.3) In most of the collisions, the molecules collide in unfavorable orientations which do not lead to reactions. 2. Describe the relationship between activation energy and the rate of reaction. (Objective 16.1.4) High activation energy means that relatively few collisions have the required energy to produce the activated complex, and the reaction rate is slow. Low activation energy means that more collisions have sufficient energy to react, and the reaction rate is faster. 3. List three factors affecting reaction rates. (Objective 16.2.1) Concentration, surface area, and temperature. 4. What does the symbol Ea represent? (Objective 16.1.4) Activation energy 5. Two molecules collide but bounce apart unchanged. What two reasons could account for their failure to react? (Objective 16.1.3) Not enough energy Unfavorable orientation 6. Explain which solid would react more quickly with a gas; a crushed sample or a whole sample. Hint: Both samples have the same mass. (Objective 16.2.1) The crushed solid would react more quickly due to increased surface area. 7. What is the role of the activated complex in a chemical reaction? (Objective 16.1.5) The activated complex allows old bonds to break and new bonds to form.