I Topic 8 notes - The University of West Georgia
advertisement

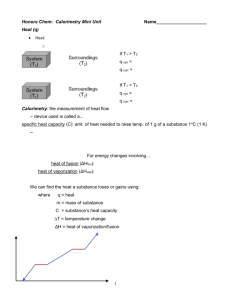
GEOL 2503 Introduction to Oceanography Dr. David M. Bush Department of Geosciences University of West Georgia Topic 8: Properties of Water POWERPOINT SLIDE SHOW NOTES 1 2 3 4 5 6 7 8 9 10 11 12 Topic 8: Properties of Water The water molecule has a chemical formula of H2O. Two hydrogen atoms and one oxygen atom. Note that the hydrogen atoms each have a positive electrical charge and the oxygen atom has a -2 negative electrical charge. So together, the total electrical charge of the molecule is zero, like most molecules. A water molecule showing the two hydrogen atoms (blue) bonded to a single oxygen atom (yellow/orange), and one of the hydrogen atoms bonded to an oxygen atom of an adjacent water molecule. Note the bond angle between the center of the oxygen atom and the two hydrogen atoms. The hydrogen atoms are not on opposite sides of the oxygen as you might think, but are pulled on one side. Water is a type of what is called a polar molecule. Although overall it has a zero electrical charge, it does have a positive side (the side with the hydrogen atoms are) and a negative side (away from the hydrogen atoms). And just like magnets, the positive side of one attracts to the negative side of the next. So water is kind of a “sticky” substance. It sticks to itself a little bit. The bonds between water molecules are called hydrogen bonds. The bonds aren’t extremely strong, but strong enough to control the behavior of water. Water has several amazing physical properties. We’ll discuss these. Properties of water—Thermal properties. Thermal properties of water include phase changes and heat capacity. “Changes of state” is just another way to say “phase change.” Water is found on Earth’s surface occurring naturally in the solid state (ice), the liquid state (liquid water), and the gaseous state (water vapor). No other substance has this property. Changing phase (or state) simply means changing from solid to liquid, liquid to gas, and so forth. Note the terms. Melting, evaporation, condensation, and freezing are common and well known terms. But what about sublimation and deposition? Sublimation means going directly from solid to gas without going through the liquid state. And deposition means going directly from gas to solid without going through the liquid state. You’ve experienced both of these. You’ve seen sublimation when we have one of our rare snow falls and it stays below freezing for a few days afterwards. Some of the snow disappears without obvious melting. It sublimates. Have you ever noticed a stray ice cube in your freezer seem to shrink over time? That’s also sublimation. On the other hand, on frosty winter mornings when you find a thin layer of ice on your windshield? That’s deposition. Water vapor in the air freezes directly onto your car. Snow cover in northern Carroll County at 8 AM on January 8, 2010. Same shot at 2 PM. Snow is gone. Some has melted but most has sublimated. How do state changes happen? Add or remove heat, break (or form) hydrogen bonds. 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Pure water is really only found in laboratories. State change temperatures are all given assuming pure water at standard air pressure. State changes go both directions. Melting and freezing points are the same temperature, just going in different directions. Same with boiling and condensation points. A well-known example of melting. Add heat. A well-known example of evaporation. Add more heat. State changes work both directions. Remove heat. Heat is not the same as temperature. What is heat? Energy. Like turning on the burner on the stove and heating a pot of soup. You add heat (energy) to the soup. Note definition of a calorie, and that 1,000 of those calories equals one food calorie. For example, we might say that an apple has 100 calories, but a nutritionist would say an apple has 100 kilocalories. We eat the apple, out body uses it as fuel. Heat is energy. Temperature is how a substance responds to adding or removing energy. One gram of water equals one cubic centimeter of liquid water. A cube of 1 cm x 1 cm x 1 cm. The size of a sugar cube. Not very much. Heating water is easy. One calorie per degree per gram. But look what happens when we change state (break hydrogen bonds). It takes 80 calories of heat to change solid water to liquid water. Not to change the temperature, just to change the state. To change liquid water to gaseous water takes 540 calories per gram! Again, not to change the temperature, just to change the state. Why does it take so much energy to change state? In reverse, the same amount of heat is released. In solid water, the molecules are linked tightly together by hydrogen bonds. In liquid water some are linked, some aren’t, and the links aren’t as tight as in ice. In gaseous water the molecules are all free. We’re really only changing how fast molecules vibrate. More heat, more vibration, bonds break. Take away heat, less vibration, bonds can form. You may have heard of “absolute zero,” the coldest possible temperature. At absolute zero, molecules cease vibrating. Absolute zero is equal to -273 degrees Celsius. Water molecule State change terminology Heat capacity. Different substances have different heat capacities. We standardize everything to water. Water has a heat capacity of 1 calorie per gram per degree Celsius. Just as we’ve been saying, it takes 1 calorie of heat to change the temperature of 1 gram of pure water by 1 degree Celsius (cal/g/ºC). 28 29 30 31 32 33 34 35 36 37 38 39 Heat capacity of common materials. Note that water’s is pretty high. Note also how low the heat capacities of metals are. Take copper, for example. It only takes 0.09 calories of energy to change the temperature of copper by 1 degree. That is why copper (and other metals) heat up so quickly and cool down so quickly. Ever grab for a wrench that has been lying in the sun for a while? Or an aluminum stirring spoon left in a pot of soup on the stove? The temperature is how the substance (copper, aluminum, water, etc.) responds to the energy being added. Graphing heat capacity. How many calories does it take to raise the temperature of 1 gram of water from -100 ºC to +150 ºC? The heat capacities of ice and water vapor are half that of liquid water. That is, it only takes adding 0.5 calories of heat to raise (or removing to lower) the temperature of ice or water vapor by one degree Celsius. So to work out the problem. Raising the temperature of ice from -100 to 0 ºC takes 50 calories. To change from ice to water takes 80 calories. Raising the temperature of liquid water from 0 to 100 ºC takes 100 calories. To change from liquid water to water vapor takes 540 calories. And to raise the temperature of water vapor from 100 to 150 ºC takes 25 calories. Added together: 50+80+100+540+25 = 795 calories. Heat capacity experiment. Three identical containers, one with water, one with dry sand, one with damp sand. Equal heat added to each with a heat lamp. Temperatures of each were noted every minute. See how the water’s temperature increases the least? Same neat added, but higher heat capacity, so lower temperature increase. Dry sand has a lower heat capacity, so its temperature raises more. Ever step on dry sand beach on a sunny summer day? It’s nice then to step onto the wet sand, right? The high heat capacity of water is critical for moving heat around the planet and having the overall effect of moderating our climate. It allows extra heat from the equator to be moved toward the poles and making Earth cooler at the equator and warmer toward the poles than it would be without water. The range of typical Earth temperatures is controlled by latent heat. Note that the surface temperatures of Earth’s land areas vary much more than ocean surface temperatures. Reflects the difference in heat capacity between land and water. Land versus ocean temperatures Evaporation is a cooling process Heat capacity summary Properties of water—cohesion. Water is “sticky.” Properties of water—surface tension Properties of water—viscosity. High viscosity means liquid flows slowly. Like the molasses in January. Or heat pancake syrup and you decrease viscosity and it flows easily. Try it at home. Properties of water—Density may be the most important properties in oceanography. Not just the density of water, but of all substances. Remember the differentiation of Earth’s interior into crust, mantle, and core? The layering is a consequence of density. More dense minerals sink to form the core, less dense float to form the crust. The definition of density is mass per unit volume. It’s measured in grams per cubic centimeter (g/cm3). We’ll come back to this many times. 40 41 42 43 44 45 46 47 48 49 50 51 52 53 What is mass? Not the same as weight. Mass is the amount of matter, weight is how gravity pulls on that mass. Pure water has a density of 1.00000 measured at a temperature of 4°C. The average density of sea water (salt water) has a density a little higher, 1.0278. Not much difference but enough to make some really wild things happen in the ocean. Remember our 1 gram of water? One cubic centimeter? It has a mass of 1 gram so its density is 1 g/cm3. The sugar cube we mentioned is denser. Same volume, but its mass is about 2.3 grams. So its density is about 2.3 g/cm3. That means a sugar cube would weigh 2.3 times as much as a cubic centimeter of water. Coke cans density experiment. Why does regular coke sink? It has sugar dissolved in it, which raises its mass, and thus its density. Effect of temperature on density Less dense substances float on denser substances The density of ice is one of the most amazing properties of water. It is less dense than liquid water. Water has its maximum density at 4°C. Colder than that the crystal structure of solid water starts to form and the molecules are actually pushed apart by the hydrogen bonds. Molecules farther apart mean less mass per unit volume, so the density is lower. That’s why ice floats. And life on Earth would be almost impossible if ice didn’t float. Complex looking graph of density versus temperature for pure water. Just follow the red line from right to left (toward lower temperatures) and notice how the line curves up (toward higher temperatures). The curve reaches its highest point (maximum density) at about 4°C (really 3.98°C but close enough). Then the solid ice crystals form and bang, the density drops all the way to 0.917, about 10% lower. That’s why ice floats only about 10% above the surface of the water. Check it out on an ice cube in your drink. You’ve heard the saying that “It’s only the tip of the iceberg?” Well, only about 10% of an iceberg shows above water. The rest of it is hidden below the water surface. Solid water is a crystal just like a mineral crystal of salt or of a gem like a ruby or emerald. Notice how long the hydrogen bonds are. Effect of salt on density. Just like sugar in the coke can experiment, sea water is denser than fresh water. Pure water has a density of exactly 1.000; fresh water’s density can vary quite a bit. Often it’s just slightly more than 1.000 Properties of water—Pressure. Pressure is the weight of something per measured area. Normal air pressure of 14.7 pounds per square inch means the weight pushing on every square inch of Earth’s surface is 14.7 pounds. Where does that weight come from? It’s the weight of the atmosphere. Imagine a column of air one inch by one inch and extending from Earth’s surface all the way into outer space. That column of air weighs 14.7 pounds (at sea level). It’s what we call standard air pressure. We standardize 14.7 pounds per square inch equal to a pressure of 1 atmosphere. Because water is much denser than air, it only takes a one-inch column of water 10 meters high to weigh 14.7 pounds. Thus, for every 10 meters of water depth, the pressure increases by 1 atmosphere. You have felt the pressure on your ears when you jump into a pool or lake. That’s the weight of the water pushing on your eardrums with a higher pressure than normal air pressure. Properties of water—Water is also known as the universal solvent. Properties of water—Light transmission The Electromagnetic Spectrum. Visible light is only a small part. Visible light doesn’t penetrate very far into seawater. 54 55 56 57 58 59 60 61 62 63 Why is the sea blue The blue part of the visible light spectrum penetrates farther into water A Secchi disk. Crude technology, but effective. Taking Secchi disk measurements. Properties of water—Sound transmission. Sound travels faster through denser materials. Sound is not transmitted in a vacuum (such as in outer space) because there is no matter to carry the sound. PDR and DSL. Deep scattering layers were recognized when precision depth records seemed to record differences of water depth at the same location between night time and day time measurements. They were recording false bottoms caused by masses of fish and small animals moving up in the water column to feed at night and down in the water column for safety during the day. PDRs. fish finders, sonars, all work on the same principle—sending sound toward the sea bottom and recording the echoes. SONAR SOFAR sound channel. Cetaceans are whales. Scientists think whales can communicate over entire oceans because their sounds are carried in Sofar channels. Variations in water depth of pressure and temperature create the Sofar channel through which sound is reflected and carries for great distances. SOFAR channel experiments. A loud noise generated near Heard Island, Australia, was detected thousands of miles away, across all oceans.