Eu 3+ -doped SiO 2 - Springer Static Content Server
advertisement

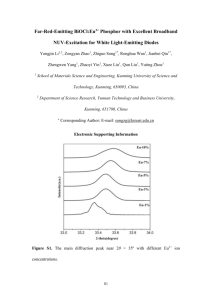
Eu3+-doped SiO2-Gd2O3 prepared by the sol-gel process: Structural and optical properties Leonardo Alves Rochaa, Marco Antonio Schiavona, Sidney José L. Ribeirob, Rogéria Rocha Gonçalvesc and Jefferson Luis Ferraria,* a –Grupo de Pesquisa em Química de Materiais – (GPQM), Departamento de Ciências Naturais, Universidade Federal de São João Del Rei, Campus Dom Bosco, 36301-160, São João Del Rei, MG, Brazil b - Instituto de Química, UNESP, P.O. Box 355, 14800-970, Araraquara, SP, Brazil c - Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, USP – Av. Bandeirantes, 3900, Ribeirão Preto/SP, 14040-901, Brazil *Prof. Dr. Jefferson Luis Ferrari Grupo de Pesquisa em Química de Materiais (GPQM) Universidade Federal de São João del-Rei Departamento de Ciências Naturais, Campus Dom Bosco Praça Dom Helvécio, 74 - Fábricas São João del-Rei – MG, Brazil Tel (Fax): +55-32-3379-2481 e-mail address: ferrari@ufsj.edu.br and jeffersonferrari@gmail.com 1 Figure Figure S1 – TGA/DTA analysis of 0.2 mol% of Eu3+-doped xerogels with ratio between Si4+:Gd3+ of A) 30:70, B) 50:50 and C) 70:30 mol%. Figure S1 shows the analysis DTA/TGA for xerogels containing 30 [Fig. S1 (A)], 50 [Fig. S1 (B)] and 70 mol% [Fig. S1 (C)] of Gd3+, and all compositions present similar behavior when compared to the xerogel with 100 mol% of Gd3+, with five steps and 45 to 50% of mass loss. 2 Figure S2 – FTIR spectra for all materials obtained at 900, 1000 e 1100 ºC heated-treated for 8h. 3 Table S3. The Infrared wavenumbers and vibration band assignments. Band Band positions (cm-1) Band assignments A 410 Gd-O stretching [1] B 470 Si-O-Si deformation [2] C 540 Gd-O stretching [1] D 800 Si-O-Si symmetric stretching [3-5] E 1079 Si-O-Si asymmetric stretching [3-5] F ~3433 OH stretching [3-5] 4 Figure S4 – SEM images of Eu3+-doped materials with ratio between Si4+:Gd3+ of (A) 100:0, (B) 30:70, (C) 50:50, (D) 70:30 and (E) 0:100 mol% heated-treated at 1000 °C for 8h. By the SEM analysis it was observed that the material mainly composed by silica [Fig. S4 (A)], and it is formed by particles with sizes on the order of micrometers, and a few plates. These plates can be related to the sintering process of the particles. The photomicrograph of the materials based on the Eu3+doped Gd2O3 [Fig. S4 (E)] showed the formation of particles of the order of nanometers. The materials with 30 [Fig. S4 (B)], 50 [Fig. S4 (C)] and 70 mol% [Fig. S4 (D)] of Gd3+ in the composition are composed by particles with intermediate shapes between materials containing only SiO2 and Gd2O3. 5 Figure S5 – EDX spectra of Eu3+-doped materials with ratio between Si4+:Gd3+ of (A) 100:0, (B) 30:70, (C) 50:50, (D) 70:30 and (E) 0:100 mol% heated-treated at 1000 °C for 8h. The decrease of the intensity of Si band located at about 1.7 KeV is clearly observed with the increase of Gd3+. It was also observed that the presence of bands around 0.8, 1.1 and 5.6 keV in all spectra, are attributed to the Eu3+, indicating the presence of RE3+ in all materials obtained. 6 Table S6. EDX quantitative analysis of the Gd3+ and Si4+ compositions. Samples Nominal quantities of Temperature (ºC) Gd3+ (mol%) 1000 0 1000 30 1000 50 1000 70 1000 100 EDX analysis Silicon (mol%) 100 69.13 49.61 30.59 0 Gadolinium (mol%) 0 30.87 50.39 69.41 100 The Table S6 shows that the compositions of the materials are in accordance to the stoichiometry proposed. 7 References: [1] N. Dhananjaya, H. Nagabhushana, B. M. Nagabhushana, B. Rudraswamy, C. Shivakumara and R. P. S. Chakradhar (2012) Spherical and rod-like Gd2O3:Eu3+ nanophosphors—Structural and luminescent properties. Bull. Mater. Sci. 35:519–527. [2] T. Liu, Y. Wang, H. Qin, X. Bai, B. Dong, L. Sun and H. Song (2011) Gd2O3:Eu3+ mesoporous SiO2 bifunctional core–shell composites: Fluorescence label and drug release. Mater. Res. Bull. 46:2296–2303. [3] M. Vallet-Regí, M. Manzano, J.M. González-Calbet and E. Okunish (2010) Evidence of drugs confinement into silica mesoporous matrices by STEM Cs corrected microscopy. Chem. Commun. 46:2956-2958. [4] R.M. Almeida, T.A. Guiton and C.G. Pantano (1990) Characterization of silica gels by infrared reflection spectroscopy.J. Non-Cryst. Solids121:193-197. [5] R.M. Almeida and C.G. Pantano (1990) Structural Investigation of silica gels films by infrared spectroscopy.J. Appl. Phys.58:4225-4232. 8