Lesson 5.7 diffusion and effusion
advertisement

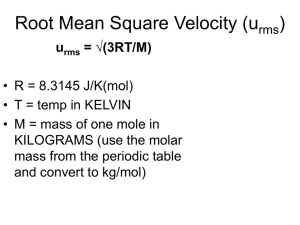
Lesson 5.7 Effusion & Diffusion Suggested Reading Zumdahl Chapter 5 Sections 5.6 & 5.7 Essential Questions What is effusion and diffusion in gases? Learning Objectives Perform calculations involving root mean square velocity. Define and differentiate effusion and diffusion. Determine the molar mass of a gas from rates of effusion. Introduction In this lesson you will be learning about Graham's law of effusion. Graham's Law shows the relationship between the molar mass of a gas and the rate at which it will effuse. Effusion is the process of gas molecules escaping through tiny holes in their container. Diffusion, such as perfume diffusing through a room, can also be considered with Graham's Law. Watch this You Tube video: https://www.youtube.com/watch?v=0uBK7VxT00E Let us first consider why gases effuse. Containers can have small holes or pores in them. Although these openings are microscopic, they are larger than the gas molecules. Randomly, the gas molecules move around the inside of the container until they impact something. This can be another molecule or the side of the container. A gas can also, instead of hitting the side of the container, pass through one of those openings by chance. This is effusion: a random movement of a gas molecule through the container's wall. A common example of this is a balloon filled with helium: first it is buoyant and floats in the air, but in a few days it hangs toward the ground or floats a few inches above the ground (if at all). The Helium has escaped through the small holes in the balloon. With Graham's Law, you can find the effusion rates for two gases or the molar mass of a gas. The gas with the lesser molar mass has a greater rate of effusion. Graham's law is derived from the KMT description of molecular speed, so before we can discuss Graham's law we must first learn something about molecular speed. Molecular Speeds Recall from KMT that gas molecules are in constant motion (assumption 2). Now we will look at the speed of molecules and at some conclusions of KMT regarding molecular speeds. According to KMT, the speeds of molecules in a gas vary over a range of values. The British physicist James Maxwell showed theoretically, and it has since been demonstrated experimentally, that molecular speeds are distributed as show in the figure. This distribution of speeds depends on the temperature. At any temperature, molecular speeds vary widely, but most are close to the average speed. As the temperature increases, the average speed increases. The root mean square velocity, urms, is a type of average molecular speed, given by the equation below (See page 204 of your textbook for the derivation of this equation). This equation shows that there is a direct relationship between the speed of a molecule and the temperature and an inverse relationship between the speed of a gas and its molar mass. Thus, when comparing two gases, the gas with the higher molar mass with have the lower rms speed. Values of rms speed calculated from this formula show that molecular speeds are astonishingly high. For example, the rms speed of H2 molecules at 20∘C is 1.90 x 103 m/s (over 4000 mi/hr!). When using this equation, be careful to use consistent units. If SI units are used then R (= 8.31 J / K ∙ mol), T (K), M (kg/mol) and the rms speed will be in m/s. Recall that the 1 J = 1 kg ∙ m2/s2. Note that in these units, H2, whose molecular weight is 2.02 amu, has a molar mass of 2.02 x 10-3 kg/mol. Example: Calculating the rms Speed of a Gas Molecules Calculate the rms speed of O2 molecules in a tank at 21∘C and 15.7 atm. Solution: The rms molecular speed is independent of pressure but does depend on the absolute temperature, which is (21 + 273) K = 294 K. To calculate u, it is best to use SI units throughout. In these units, the molar mass of O2 is 32.0 x 10-3 kg/mol, and R = 8.31 J / K ∙ mol. Hence, Effusion Recall that effusion is the process in which a gas flows through a small hope in a container. Thomas Graham was the first to study effusion and he discovered in 1946 that the rate of effusion of a gas is inversely proportional to the square root of the molecular weight of the gas at constant temperature and pressure. Stated another way, the relative rates of effusion of two gases at the same temperature and pressure are given by the inverse ratio of the square roots of the molar masses of the gases. This is called Graham's law of effusion and is expressed mathematically as This equation is derived from the equation for rms speed, and the derivation can be found on page 207 of your textbook. Thus, the KMT agrees with the experimental results for effusion. Diffusion When you bake cookies, you can usually smell the wonderful aroma throughout the house. The spread of an odor is easily explained with KMT. All molecules are in constant, random motion, and eventually a cluster of molecules of a particular substance will spread out to occupy a larger and larger space. Gaseous diffusion is the process whereby a gas spreads out through another gas to occupy the space uniformly. When you think about the KMT calculations of rms you might wonder why it takes a gas minutes rather than seconds to diffuse. This was, in fact, one of the first major criticisms of KMT. The answer is simply that a molecule never travels very far in one direction before it collides with another molecule and moves off in another direction. This results in a zig-zagging path that requires the molecule to travel much further than the straight-line distance. Although the rate of diffusion certainly depends in part on the rms speed, the effect of molecular collisions makes the theoretical picture somewhat complicated. However, Graham's law of effusion can be used to approximate rates of diffusion. The following video discusses Graham's law of effusion and diffusion as though they were synonymous. Although this is common, this in not strictly correct. Graham's law describes rates of effusion, and is used approximate rates of diffusion. Watch this You Tube video: https://www.youtube.com/watch?v=FCVjyS4bQwg HOMEWORK: Refer to the power point notes examples for effusion examples. Practice exercise 10.15