Science Jeopardy: Gas Laws & Properties Review Game
advertisement

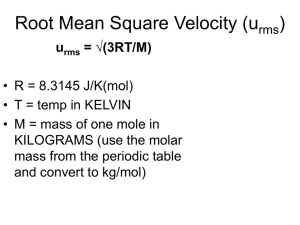
Science Jeopardy! Properties of gases Boyle’s+ charle’s Laws GayLussac’s+ combined gas laws Ideal gas laws Dalton’s+ Graham’s Laws Vocab CH14 100 100 100 100 100 100 200 200 200 200 200 200 300 300 300 300 300 300 400 400 400 400 400 400 500 500 500 500 500 500 100 What is compressibility? A measure of how much the volume of matter decreases under pressure. 200 Why are gases easily compressed? Because of the spaces between the particles in the gas. 300 Name 3 factors that affect gas pressure. Gas, volume and temparature 400 How can you raise the pressure exerted by a contained gas? By reducing its volume 500 If the temperature is constant, as the pressure of the gas increases, what happens to the volume? The volume decreases. 100 What is Boyle’s law? For a given mass of gas in a constant temperature, the volume of the gas there is inversely with pressure. 200 What is the equation of the Boyle’s Law? P1xV1= P2xV2 300 What is Charles's Law? The volume of fix mass of gas is directly proportional to its kelvin temperature if the pressure is kept constant. 400 What is the equation of Charles’s law? V1/T1=V2/T2 500 If a sample of gas occupies 6.8L at 325c, what will its volume be at 25c if the pressure does not change. 3.38 100 What does Gay-Lussac’s Law state? The pressure of a gas is directly proportional to the kelvin temperature if the volume remains constant. 200 What is the equation is Gay-lussac’s law? P1/T1=P2/T2 300 What is the combined gas law? The combined gas law allows you to do calculations for situations in which only the amount of gas is constant. 400 What is the equation of the combined gas law? p1xV1/T1=p2xV2/T2 500 A cylinder with a moveable piston contains 198 mL of nitrogen gas at a pressure of 1.22 atm and a temperature of 302 K. What must the final volume be for the pressure of the gas to be 1.52 atm atm at a temperature of 370 K? Vfinal =___?___ mL T2P1V1 / P1T1 = V2 (370K)(1.22atm)(198mL) / (1.52atm)(302K) = V2 89.3772 / 459.04 = V2 194.704mL = V2 Answer - 195mL (Rounded up due to proper amount of significant figures) 100 What is the ideal gas law? The ideal gas law is the equation of state of a hypothetical ideal gas. 200 Given the following sets of values, calculate the unknown quantity. a) P = 1.01 atm V=? n = 0.00831 mol T = 25°C 0.477 L 300 What is the volume of 1.00 mole of a gas at standard temperature and pressure? 22.4 L 400 When filling a weather balloon with gas you have to consider that the gas will expand greatly as it rises and the pressure decreases. Let’s say you put about 10.0 moles of He gas into a balloon that can inflate to hold 5000.0L. Currently, the balloon is not full because of the high pressure on the ground. What is the pressure when the balloon rises to a point where the temperature is -10.0°C and the balloon has completely filled with the gas. 73 L 500 You decide to go on a long hot air balloon ride, so you decide to bring some shampoo to wash your hair with. However there is some gas inside the shampoo bottle when you start to climb into the basket at the beginning of your journey. In fact, because you are a good scientist you decide to constantly take measurements of your surroundings. The shampoo bottle contains 435ml of gas, under a pressure of 1.10 atm, at a temperature of 30.0°C. When you climb high into the air the bottle starts to expand eventually exploding and covering you and your companions with Pert Plus. Eager to explain this phenomenon you take some measurements: the pressure, you note, has dropped to 0.734 atm and the temperature has dropped to 5.00°C. To what new volume did the gas inside the bottle expand? 598 mL 100 What is Dalton’s law of partial pressures? At constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressure of the component gases. 200 What is graham’s law? known as Graham's law of effusion, was formulated by Scottish physical chemist Thomas Graham in 1846. Graham found experimentally that the rate of effusion of a gas is inversely proportional to the square root of the mass of its particles. 300 What is the equation of the graham’s law 400 What is diffusion? Diffusion is one of several transport phenomena that occur in nature. 500 What is effusion effusion is the process in which individual molecules flow through a hole without collisions between molecules. 100 What is partial pressure Contribution each gas makes to the total pressure 200 What is gas? One of the three states of liquid 300 What is volume? Volume is the quantity of threedimensional space enclosed by some closed boundary 400 What is the unit for pressure? kpa 500 What is the unit for volume? liters