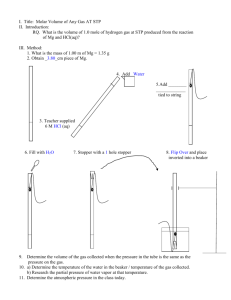
The Gas Laws
advertisement
GASES Name:______________________________________________Period:____Date:____ I. PHYSICAL PROPERTIES A. Real Gases vs. Ideal Gases: Ideal gas = an imaginary gas that conforms perfectly to all the assumptions of the kineticmolecular theory • We will assume that the gases used for the gas law problems are ideal gases. Real gas = a gas that does not behave completely according to the assumptions of the kineticmolecular theory. • All real gases deviate to some degree from ideal gas behavior. However, most real gases behave nearly ideally when their particles are sufficiently far apart and have sufficiently high kinetic energy. Causes of non-ideal behavior: • The kinetic-molecular theory is more likely to hold true for gases whose particles have NO attraction for each other. 1. High pressure (low volume): • Space taken up by gas particles becomes significant • Intermolecular forces are more significant between gas particles that are closer together 2. Low temperature: • Gas particles move slower so intermolecular forces become more important B. Kinetic Molecular Theory- Ideal Gases Kinetic Molecular Theory (KMT) = the idea that particles of matter are always in motion and that this motion has consequences. • theory developed in the late 19th century to account for the behavior of the atoms and molecules that make up matter • based on the idea that particles in all forms of matter are always in motion and that this motion has consequences • can be used to explain the properties of solids, liquids, and gases in terms of the energy of particles and the forces that act between them KMT describing particles in an IDEAL gas: • have no volume. • have elastic collisions. • are in constant, random motion. • don’t attract or repel each other. • have an avg. KE directly related to Kelvin temperature. C. Kinetic Molecular Theory- Real Gases KMT describing particles in a REAL gas: • have their own volume • attract each other 1 • • • Gas behavior is most ideal… at low pressures at high temperatures in nonpolar atoms/molecules D. Characteristics of Gases- using KMT Gases expand to fill any container. • KMT - gas particles move rapidly in all directions without significant attraction or repulsion between particles Gases are fluids (like liquids). • KMT - No significant attraction or repulsion between gas particles; glide past each other Gases have very low densities. • KMT - particles are so much farther apart in the gas state Gases can be compressed. • KMT - gas particles are far apart from one another with room to be “squished” together Gases undergo diffusion & effusion. • KMT – gas particles move in continuous, rapid, random motion E. Temperature Always use Kelvin temperature when working with gases. ºC = 5/9 (ºF – 32) K = ºC + 273 F. Pressure pressure force area Why do gases exert pressure? • Gas particles exert a pressure on any surface with which they collide! o More collisions = increase in pressure! Barometer = measures atmospheric pressure • The height of the Hg in the tube depends on the pressure • The pressure of the atmosphere is proportional to the height of the Hg column, so the height of the Hg can be used to measure atmospheric pressure! Pressure UNITS • 101.3 kPa (kilopascal) • 1 atm • 760 mm Hg • 760 torr G. STP Standard Temperature & Pressure 0°C (exact) 1 atm (exact) 273 K 101.3 kPa 760 mm Hg (exact) 760 torr (exact) 2 II. THE GAS LAWS GAS LAW PROBLEMS - MUST USE KELVIN! K = ºC + 273 A. Boyle’s Law The pressure and volume of a gas are INVERSELY related • at constant mass & temp PV = k P V Boyle’s Law: P1V1 = P2V2 Real life application: When you breathe, your diaphragm moves downward, increasing the volume of the lungs. This causes the pressure inside the lungs to be less than the outside pressure so air rushes in. Ex: Halving the volume leads to twice the rate of collisions and a doubling of the pressure. As the volume increases, the pressure decreases! B. Charles’ Law The volume and temperature (K) of a gas are DIRECTLY related • at constant mass & pressure V k T V T Real life application: Bread dough rises because yeast produces carbon dioxide. When placed in the oven, the heat causes the gas to expand, and the bread rises even further. Charles’ Law: V1 = V2 T1 T2 3 As temperature decreases, the volume of the gas decreases Liquid nitrogen’s temp. is about 63K or -210ºC or -346ºF! As temperature decreases, the volume of the gas decreases NUMBER OF PARTICLES & PRESSURE ARE CONSTANT!! C. Guy-Lussac’s Law P k T P T • The pressure and temperature (K) of a gas are DIRECTLY RELATED • at constant mass & volume • Real life application: The air pressure inside a tire increases on a hot summer day. • Guy-Lussac’s Law: • When the temp. of a gas increases (KE increases) and gas particles move faster and hit container walls more frequently and collisions are more forceful P1 = T1 P2 T2 4 D. Combined Gas Law • Combined Gas Law: P1 V1 T1 = P2V2 T2 E. EXAMPLE Problems: Show all work & units. Identify which gas law will be used and then list all the variables. Finally solve the gas law equation for the variable that you are looking for and solve. 1.) A gas occupies 473 cm3 at 36°C. Find its volume at 94°C. Which law?____________________________ 2.) A gas occupies 100. mL at 150. kPa. Find its volume at 200. kPa. Which law?____________________________ 3.) A gas occupies 7.84 cm3 at 71.8 kPa & 25°C. Find its volume at STP. Which law?____________________________ 4.) A gas’ pressure is 765 torr at 23°C. At what temperature will the pressure be 560. torr? Which law?____________________________ 5 III. STANDARD MOLAR VOLUME, GAS DENSITIES & MOLAR MASS A. Standard Molar Volume = The volume occupied by one mole of a gas at STP 22.41410 L/mol or about 22.4 L/mol In other words, one mole of any ideal gas at STP will occupy 22.4 L B. Example Problems- Standard Molar Volume 1) A chemical reaction is expected to produce 0.0680 mol of oxygen gas. What volume of gas in L will be occupied by this gas sample at STP? 2) A chemical reaction produced 98.0 mL of SO2 at STP. What mass (in grams) of the gas was produced? C. Density = The ratio of an object’s mass to its volume. D = M V The volume (and density) of a gas will change when pressure and temperature change. Densities are usually given in g/L at STP For an ideal gas: Density (at STP) = molar mass standard molar volume D. Example Problems- density 1) What is the density of CO2 in g/L at STP? 2) What is the molar mass of a gas whose density at STP is 2.08 g/L. 6 IV. MORE GAS LAWS: IDEAL GAS LAW & AVOGADRO’S LAW A. Avogadro’s Law = Equal volumes of gases contain equal numbers of moles • at constant temp & pressure V n V k n B. Ideal Gas Law Boyle’s, Charles, and Avogadro’s laws are contained within the ideal gas law! Ideal Gas Law: PV = nRT • P = pressure • V = volume • n = moles • T = temperature (in K) • R = the ideal gas constant Gas Constant: • R = 0.0821 L ∙ atm mol ∙ K • R = 8.314 L ∙ kPa mol ∙ K • R = 62.4 L ∙ mm Hg mol ∙ K • R= 8.315 dm3 kPa mol K You don’t need to memorize these! C. Ideal Gas Law Problems: PV = nRT 1.) Calculate the pressure in atmospheres of 0.412 mol of He at 16°C & occupying 3.25 L. 2.) Find the volume of 85 g of O2 at 25°C and 104.5 kPa. 7 3.) At 28C and 98.7 kPa, 1.00 L of an unidentified gas has a mass of 5.16 g. Calculate the number of moles of gas present and the molar mass of the gas. V. MORE GAS LAWS: DALTON’S LAW & GRAHAM’S LAW A. Dalton’s Law = The total pressure of a mixture of gases equals the sum of the partial pressures of the individual gases. Ptotal = P1 + P2 + P3 … Dalton’s Law: When a H2 gas is collected by water displacement, the gas in the collection bottle is actually a mixture of H2 AND water vapor. 1.) Hydrogen gas is collected over water at 22.5°C. Find the pressure of the dry gas if the atmospheric pressure is 94.4 kPa. 2.) A gas is collected over water at a temp of 35.0°C when the barometric pressure is 742.0 torr. What is the partial pressure of the dry gas? 8 B. Graham’s Law Diffusion = Spreading of gas molecules throughout a container until evenly distributed. Effusion = Passing of gas molecules through a tiny opening in a container Speed of diffusion/effusion Kinetic energy is determined by the temperature of the gas. At the same temp & KE, heavier molecules move more slowly. o Larger m smaller v KE = ½mv2 KE = kinetic energy m= molar mass v = velocity Graham’s Law = Rate of effusion of a gas is inversely related to the square root of its molar mass. The equation shows the ratio of Gas A’s speed to Gas B’s speed. vA vB mB mA C. Graham’s Law Problems 1.) Determine the relative rate of effusion for krypton and bromine. The first gas is “Gas A” and the second gas is “Gas B”. Relative rate mean find the ratio “vA/vB”. Kr effuses 1.381 times faster than Br2. 2.) A molecule of oxygen gas has an average speed of 12.3 m/s at a given temp and pressure. What is the average speed of hydrogen molecules at the same conditions? 3.) An unknown gas effuses 4.0 times faster than O2. Find its molar mass. The first gas is “Gas A” and the second gas is “Gas B”. The ratio “vA/vB” is 4.0. 9 VI. GAS STOICHIOMETRY A. Gas Stoichiometry Moles Liters of a Gas: • STP - use 22.4 L/mol • Non-STP - use ideal gas law Non-STP • Given liters of gas? start with ideal gas law • Looking for liters of gas? start with stoichiometry conv. B. Gas Stoichiometry Problem: Volume Mass Thinking: o Gas volume A moles A moles B mass B If gas “A” is at STP: ? L “A” 1 mol “A” ? mol “B” molar mass (g) “B” 22.4 L “A” ? mol “A” 1 mol “B” If gas “A” is NOT at STP: o Use PV = nRT to find moles of “A” ? mol “A” ? mol “B” ? mol “A” molar mass (g) “B” 1 mol “B” 1) How many grams of Al2O3 are formed from 15.0 L of O2 at 97.3 kPa & 21°C? 2) What mass of sulfur is required to produce 12.6 L of sulfur dioxide at STP according to the equation: S8 (s) + 8 O2 (g) 8 SO2 (g) 10 C. Gas Stoichiometry Problem: Mass Volume Thinking: o Mass A moles A moles B gas volume B If gas “B” is at STP: ? g “A” 1 mol “A” ? mol “B” 22.4 L “B” molar mass (g) “A” ? mol “A” 1 mol “B” If gas “B” is NOT at STP: ? g “A” 1 mol “A” molar mass (g) “A” o ? mol “B” ? mol “A” = ? mol “B” Then use PV = nRT to find volume of “B” 1) What volume of chlorine gas at STP is needed to react completely with 10.4 g of sodium to form NaCl? 2) What volume of CO2 forms from 5.25 g of CaCO3 at 103 kPa & 25ºC? 11 Gas Law Practice Problems: Boyle’s, Charles’, Guy-Lussac’s, & Combined Directions: 1) List all the variables along the left hand side for EACH problem 2) Solve the formula for the variable that you used for EACH problem 3) SHOW all work & proper UNITS in order to receive full credit Remember: Convert all temperatures to Kelvin! 1) Helium occupies 3.8 L at -45°C. What volume will it occupy at 45°C? Which law?_____________________________ 2) Ammonia gas occupies a volume of 450. mL at 720. mm Hg. What volume will it occupy at standard pressure? Which law?_____________________________ 3) A gas at STP is cooled to -185°C. What pressure in atmospheres will it have at this temperature (volume remains constant)? Which law?_____________________________ 12 4) A gas occupies 1.50 L at 850. mm Hg and 15°C. At what pressure will this gas occupy 2.50 L at 30.°C? Which law?_____________________________ 5) Chlorine gas has a pressure of 1.05 atm at 25°C. What pressure will it exert at 75°C? Which law?_____________________________ 6) A gas occupies 256 mL at 720. torr and 25°C. What will its volume be at STP? Which law?_____________________________ 7) At 27°C, fluorine occupies a volume of 500. mL. To what temperature in degrees Celsius should it be lowered to bring the volume to 200. mL? Which law?_____________________________ 13 8) A gas occupies 125 mL at 125 kPa. After being heated to 75°C and depressurized to 100.0 kPa, it occupies 0.100 L. What was the original temperature of the gas? Which law?_____________________________ 9) A 3.20 L sample of gas has a pressure of 102 kPa. If the volume is reduced to 0.650 L, what pressure will the gas exert? Which law?_____________________________ 10) A gas at 2.5 atm and 25°C expands to 750 mL after being cooled and depressurized to STP. What was the original volume of the gas? Which law?_____________________________ 14 Practice Problems: Standard Molar Volume, Gas Density & Molar Mass Directions: Solve each of the following problems. Show all work. Units should be included on all numbers. 1) A sample of hydrogen gas occupies 14.1 L at STP. How many moles of the gas are present? 2) At STP, 1.33 x 104 mL of chlorine gas is produced during a chemical reaction. What is the mass of the gas? 3) What is the volume of 77.0 g of nitrogen dioxide gas at STP? 4) What is the density of SO2 gas in g/L at STP? 5) A 0.519 g gas sample is found to have a volume of 0.200 L at STP. What is the molar mass of this gas? 15 6) What is the molar mass of a gas if a 1.25 g sample of the gas has a volume of 322 mL at STP? 7) Calculate the density, in g/L, of each of the following gases at STP: a) nitrogen gas b) carbon monoxide gas c) sulfur dioxide gas d) ammonia gas, NH3 8) At STP, what is the volume, in liters, of 7.08 mol of nitrogen gas? 16 9) What is the molar mass of a gas that has a density of 1.28 g/L at STP? 10) A 3.25 g gas sample is found to have a volume of 2.30 L at STP. What is the molar mass of this gas? 11) A sample of neon gas occupies 550. mL at STP. Calculate the mass, in grams, of the neon gas. 12) Suppose you need 4.22 g of chlorine gas. What volume, in milliliters, at STP would you need to use? 13) At STP, 3.00 L of water vapor (gaseous water) is produced during a chemical reaction. Calculate the mass of the gas. 17 14) How many moles are contained in 0.125 L of neon gas at STP? 15) What is the molar mass of a gas whose density at STP is 1.33 g/L. 16) What mass of chlorine gas is contained in a 10.0 L tank at 27C and 3.50 atm? 17) What is the mass, in grams, of oxygen gas in a 12.5 L container at 45C and 7.22 atm? 18 18) Calculate the mass, in grams, of a sample of carbon dioxide gas in a 250. mL container at 27C and 771 mm Hg. 19) What is the molar mass of a gas if 0.427 g of the gas occupies a volume of 0.125 L at 20.C and 99.2 kPa? 20) Calculate the pressure, in kPa, exerted by 0.325 mol of hydrogen gas in a 4.08 L container at 35C. 19 21) A 16.0 g sample of gas was found to occupy 8.00 L at 1.50 atm and 27C. Calculate the molar mass of the gas. 22) How many liters are occupied by 0.909 mol of nitrogen gas at 125C and 0.910 atm? 23) Calculate the mass, in grams, of a sample of oxygen gas in a 500. mL container at 31C and 745 mm Hg. 20 24) A chemistry student determines the mass of a sample of gas to be 1.25 g. Its volume is 1.00 L at a temperature of 27C and a pressure of 99.9 kPa. Calculate the molar mass of the gas. 25) A chemist determines the mass of a sample of gas to be 3.17 g. Its volume is 942 mL at a temperature of 14C and a pressure of 828 mm Hg. Calculate the molar mass of the gas. 21 More Gas Law Practice Problems: Dalton’s Law & Graham’s Law Directions: Solve each of the following problems. Show all work. Units should be included on all numbers. Water-Vapor Pressure Table on the” gas formula sheet.” 1) Nitrogen is collected over water at 21.5C. What is the partial pressure of nitrogen if the atmospheric pressure is 99.4 kPa? 2) What is the relative rate of effusion of NO2 and CH4? 3) Does NO2 effuse faster or slower than CH4? ______________ Why? 4) Argon is collected over water at 30C. Find the pressure of the dry gas if the barometric pressure is 0.8450 atm. 5) Carbon dioxide molecules have an average speed of 25.0 m/s at a given temperature and pressure. What is the average speed of carbon monoxide molecules at the same conditions? 6) An unknown gas effuses 0.250 times as fast as helium. What is its molar mass? 22 Practice Problems: Gas Stoichiometry Directions: Solve each of the following problems. Show all work. Units should be included on all numbers. 1) Calcium carbonate, also known as limestone, can be heated to produce calcium oxide (lime), an industrial chemical with a wide variety of uses. The balanced equation is as follows: ∆ CaCO3(s) CaO(s) + CO2(g) How many grams of calcium carbonate must be decomposed to produce 5.00 L of carbon dioxide at STP? 2) Aluminum granules are a component of some drain cleaners because they react with sodium hydroxide to release both heat and gas bubbles, which help clear the drain clog. What mass of aluminum is needed to produce 4.00 L of hydrogen gas at STP? The balanced chemical equation follows: 2 NaOH(aq) + 2 Al(s) + 6 H2O(l) 2 NaAl(OH)4(aq) + 3 H2(g) 3) How many grams of aluminum metal must react with aqueous sodium hydroxide to produce 7.50 L of hydrogen gas collected at 22C and 100. kPa if the other product is aqueous Na3AlO3? 4) Write a balanced chemical equation for the decomposition of solid potassium chlorate. Determine the mass, in grams, of potassium chlorate needed to produce 9.28 L of oxygen gas collected at 0.987 atm and 25C. 23 5) Write a balanced chemical equation for a reaction between solid magnesium metal and oxygen gas that produces solid magnesium oxide. How many grams of magnesium oxide can be produced if 2.22 L of oxygen gas react at 23C and 745 mm Hg? 6) Assume that 5.60 L of H2 gas at STP react with CuO according to the following equation: CuO(s) + H2(g) Cu(s) + H2O(g) a) Balance this equation. b) Calculate the mass of CuO needed for this reaction. c) How many grams of EACH product can be produced? 7) Solid iron(III) hydroxide decomposes to produce iron(III) oxide and water vapor. If 0.75 L of water vapor is produced at STP: a) How many grams of iron(III) hydroxide are used? b) How many grams of iron(III) oxide can be produced? 24 8) Assume 14.0 L of SO2 gas at 725 mm Hg and 37C react according to the following equation: SO2(g) + H2O(l) H2SO3(aq) a) Balance this equation. b) How many grams of H2O are required? c) How many grams of H2SO3 can be produced? 9) Assume that 8.50 L of I2 gas are produced using the following reaction that takes place at STP: KI(aq) + Cl2(g) KCl(aq) + I2(g) a) Balance this equation. b) How many grams of KI are needed? c) How many grams of KCl can be produced? 25 10) If 45.0 L of methane, CH4, undergoes complete combustion at 730. mm Hg and 20.C, how many grams of EACH product can be formed? 11) How many liters of gaseous carbon monoxide at STP can be produced from the burning of 65.5 g of carbon according to the following equation? 2 C(s) + O2(g) 2 CO(g) 12) Write a balanced chemical equation for the reaction between sodium metal and liquid water. When 4.60 g of sodium react, how many liters of hydrogen gas at STP can be produced? 26 13) Tungsten, a metal that is used in light bulb filaments, is produced industrially by the following reaction of tungsten oxide with hydrogen: WO3(s) + 3 H2(g) W(s) + 3 H2O(l) How many liters of hydrogen gas at 35C and 745 mm Hg are needed to react completely with 875 g of tungsten oxide? 14) Air bags in cars are inflated by the sudden decomposition of sodium azide, NaN3, according to the following equation: 2 NaN3(s) 3 N2(g) + 2 Na(s) What volume of nitrogen gas at 131.4 kPa and 87C can be produced by the reaction of 70.0 g of sodium azide? 27 15) Write a balanced chemical equation for the reaction between aluminum metal and hydrochloric acid. Calculate the volume, in liters, of hydrogen gas at 1.15 atm and 33C that can be produced if 13.5 g of aluminum are consumed. 16) Given: Na(s) + Cl2(g) NaCl(s) a) Balance this equation. b) What volume of chlorine gas at 38C and 1.63 atm is needed to react completely with 10.4 g of sodium? 28 17) Assume that 13.5 g of aluminum react with hydrochloric acid according to the following equation, at STP: Al(s) + HCl(aq) AlCl3(aq) + H2(g) a) Balance this equation. b) What volume of hydrogen gas can be produced at STP? 18) Carbon reacts with oxygen gas according to the following equation: C(s) + O2(g) CO(g) a) Balance this equation. b) How many liters of carbon monoxide gas at 27C and 115.5 kPa can be produced from the burning of 65.5 g of carbon? c) How many liters of oxygen gas at 27C and 115.5 kPa are required for the burning of 65.5 g of carbon? 19) How many liters of hydrogen gas at STP can be produced by the reaction of sodium with 3.20 g water? (Write the chemical equation first.) 29 GASES CONCEPT REVIEW QUESTIONS: 1) Define kinetic-molecular theory (KMT). 2) List the five assumptions of the kinetic-molecular theory of IDEAL gas particles. a) b) c) d) e) 3) At what conditions do gases behave nearly ideal? _______________________________________ 4) List five characteristics of gases that can be explained in terms of KMT. a) b) c) d) e) 5) What characteristics do real gases have that contradict the assumptions of kinetic-molecular theory? 6) What conditions lead to the least ideal behavior? Why? 7) What four quantities are needed to accurately describe a gas? 30 8) Define pressure. 9) How does changing the area of contact affect the amount of pressure exerted by an object? 10) Describe how gas pressure is produced. 11) A device used to measure atmospheric pressure is called a _____________________________. 12) How are atmospheric pressure and the height of mercury in a barometer related? 13) What conditions are indicated by STP? 14) State Boyle’s Law and include two variables that are held constant. Represent Boyle’s Law graphically and state whether this is a direct or inverse relationship. 15) State Boyle’s Law using a mathematical equation. ________________________________ 16) Use KMT to explain Boyle’s Law. 17) State Guy-Lussac’s Law and include two variables that are held constant. Represent Guy-Lussac’s Law graphically and state whether this is a direct or inverse relationship. 31 18) State Guy-Lussac’s Law using a mathematical equation. ________________________________ 19) Use KMT to explain Guy-Lussac’s Law. 20) State Charles’ Law and include two variables that are held constant. Represent Charles’ Law graphically and state whether this is a direct or inverse relationship. 21) State Charles’ Law using a mathematical equation. ________________________________ 22) Define standard molar volume. 23) State Avagadro’s Law and include two variables that are held constant. 24) State Dalton’s Law. 25) State Graham’s Law. 26) What is the difference between diffusion and effusion? 27) What types of gas molecules typically diffuse and effuse faster? 28) List the four common units of pressure and their relationship to 1 atmosphere (atm). 32 GAS PROBLEMS REVIEW QUESTIONS: Directions: Show all work & units. Identify which gas law will be used and then list all the variables. Finally solve the gas law equation for the variable that you are looking for and solve. 1) Hydrogen is collected over water at 0.9750 atm and 28C. What is the partial pressure of H2? 2) How many moles of chloroform, CHCl3, are required to fill a 253-mL flask at 100.C and 940. mm Hg? 3) You want the pressure inside a bottle to be 75.0 kPa at 23C. At what temperature should you seal the bottle when the pressure is 113.46 kPa? 4) A diver’s lungs hold about 20.0 L of air underwater at a pressure of 875 mm Hg. Assuming he holds his breath and his lungs don’t burst, what will be the volume of air in his lungs at standard pressure on the water’s surface? 5) What pressure is required to compress a gas that occupies 655 L at 25C and 1.0 atm to a volume of 40.0L at 18C? 33 6) Oxygen gas effuses how many times faster than sulfur dioxide? 7) What is the molar mass of an unknown gas if it effuses 0.906 times as fast as argon? 8) A shampoo bottle contains 443 mL of air at 65C. What is its volume when it cools to 22C? 9) A balloon is filled with helium to a volume of 12.5 liters at 25C and 101 kPa. How many grams of helium are in the balloon? 10) At a certain temperature and pressure, chlorine molecules have an average speed of 0.0380 m/s. What is the average speed of SO2 molecules under the same conditions? 34 11) What is the molar mass of a gas if 0.271 g of the gas occupies a volume of 0.175 L at 23C and 87.4 kPa? Directions: Solve each of the following problems. Show all work. Units should be included on all numbers. 12) What is the density of CO gas in g/L at STP? 13) What is the molar mass of a gas whose density at STP is 2.86 g/L? 14) A 0.837 g gas sample is found to have a volume of 0.400 L at STP. What is the molar mass of this gas? 15) A sample of argon gas occupies 732 mL at STP. Calculate the mass, in grams, of the argon gas. 16) How many moles are contained in 0.276 L of helium gas at STP? 35 Directions: Solve the following gas stoichiometry problems. Show all work. Units should be included on all numbers. 17) What volume of chlorine is required to produce 25.4 g of copper(II) chloride at STP? Use the following balanced equation: Cu + Cl2 CuCl2 18) At 778 mm Hg and 25C, how many grams of zinc are required to produce 25.2 liters of hydrogen gas? Balance the following equation: Zn + HCl ZnCl2 + H2 19) If 5.45 g of potassium chlorate decompose, how many liters of oxygen gas are given off at 1.58 atm and 32C? Balance the following equation: KClO3 KCl + O2 36 20) When aluminum is burned in 15.0 L of oxygen at STP, how many grams of aluminum oxide are formed? 21) If 12.8 g of CaCO3 decomposes at 38C and 0.96 atm, how many liters of CO2 are formed in addition to CaO? Use the following balanced equation: CaCO3 CaO + CO2 22) What mass of glucose (C6H12O6) is required to produce 150. mL of carbon dioxide at 102 kPa and 23C? Use the following balanced equation: C6H12O6 + 2O2 2CH3COOH + 2CO2 + 2H2O 37 GASES TEST STUDY GUIDE Chapter 10 (pg 382-423) Read over your notes and rework your class/homework assignments. A “Gas Formula Sheet” will be given to you to use on the test. You will do “Mole-tastic” on this test if you can do the following things. PHYSICAL PROPERTIES OF GASES Identify the 5 assumptions of Kinetic Molecular Theory. Explain the difference between real and ideal gases. What characteristics of real gases conflict with KMT? What conditions favor ideal gas behavior and why? Identify the common characteristics of gases & explain using KMT. Define STP and make temperature and pressure conversions. THE GAS LAWS Define Boyle’s Law, Charles’ Law, & Guy-Lussac’s Law: Which properties are involved and what is their relationship? Graphical analysis of the relationships Calculate and predict using the gas laws- Boyle’s Law, Charles’ Law, Guy-Lussac’s Law & Combined Gas Law. IDEAL GAS LAW Define Avogadro’s Principle. Solve problems using the Ideal Gas Law. STANDARD MOLAR VOLUME, GAS DENSITY, MOLAR MASS CALCULATIONS Define standard molar volume Solve problems for density, volume and molar mass. For an ideal gas: Density (at STP) = molar mass standard molar volume DALTON’S LAW & GRAHAM’S LAW Define diffusion, effusion, Dalton’s Law, and Graham’s Law. Solve gas collection problems using Dalton’s Law. Use Graham’s Law to find… the relative rate of diffusion for two gases. the average speed of a gas. the molar mass of an unknown gas. GAS STOICHIOMETRY AT NON-STP & STP Solve gas stoichiometry problems at non-STP & STP. Volume mass Mass Volume 38 Gas Formula Sheet STP: exactly 0°C and 1 atm (760 mm Hg) 1 atm = 760 mm Hg (exact conversion) 1 atm = 760 torr (exact conversion) 1 atm = 1.013 x 105 Pa Standard molar volume = 22.4 L/mol (at STP) P1V1 = P2V2 P1 V1 T1 = V1 T1 P2V2 T2 = V2 T2 vA vB P1 = P2 T1 T2 1 atm = 101.3 kPa Ptotal = P1 + P2 + P3 … mB mA PV = nRT R = 0.0821 L ∙ atm mol ∙ K R = 8.314 L ∙ kPa mol ∙ K R = 62.4 L ∙ mm Hg mol ∙ K R= 8.315 dm3 kPa mol K 39