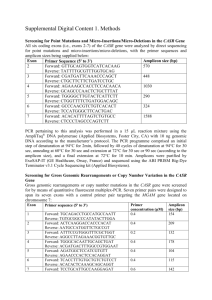
Supplementary materials Table 1 Oligonucleotide primers for PCR
advertisement

Supplementary materials Table 1 Oligonucleotide primers for PCR amplification of UMOD Exon Sequence (5'3') forward GAGTACAAGGGGTAAAGTCCCAGCAAAA reverse AAGTGCGATAGGAGGATTGAGATCACCT forward AGTTCCCTGGAGAATGAGGGAAGGATCT reverse CAGGCGTAGCCCTCCCCGTACT forward ATGTGTCAATGTGGTGGGCAGCTACTT reverse GGTCACAGGGACAGACAGACAATCAATA forward GTACTGCACAGGTCAGCCGGAGTCT reverse GTGGATCTTCTGTTTTCACTCAGGTTGG forward CTCAAGGCTATGCTGAGCACTTCCAGAT reverse AAACTGAGGCACAGCCAGGCTAAATAAC forward CCTACTCAGATTCTCATGCCCCTTTCTC reverse AGTCTAGATTTGAACCTGGGCTCCAGAA forward CAACAGTTGGTGGGTTCCACTCTTATTG reverse AAAGATGCAATTTTCCTCCATCCAAGTC forward TATCTTATCACATGGGCTGCGATCATTT reverse TCTGGTAAAAGAGGGAAACAGGGAAGAA forward TATCTAACAAATGGCAGAGCTGGGACCT reverse CCAATGAATGGGTGTATGAATGGGAATA forward TGGCTGCGAAGTGAAAAGTAGATTGAAG reverse ACTCAGGTTCTCTGAGCCACTCTCCTTA forward GTGTCCTCTTCTGATTGGTCAGCCTAGA reverse GTCACAAGTCCCATTTTGAGAAAAAGCA 2 3a 3b 4 5 6 7 8 9 10 11 1 Fig.S1 The novel mutation of uromodulin C112Y found in the FJHN family. (A) The family pedigree of FJHN patients. Family members with symptoms are indicated by a blackened within the symbol. † = death, N.E. = not examined. (B) Partial sequence electropherogram of UMOD gene of the propositus (upper panel) and schematic diagram of uromodulin structure (lower panel). The C112Y mutation located in the Ca-binding site of EGF- like domain encoded in the exon 3. UMOD gene consists of 11 exons. Exon 2-11 are coding, and exon 1 is UTR. EGF = epidermal growth factor-like domain, cb = calcium binding motif, CCS = consensus cleavage site, GPI = glycosyl-phospatidylinositol-anchoring site, D8C = the domain of eight cysteines, ZP-N = zona pellucida-N subdomains, ZP-C = zona pellucida-C subdomains, IHP = internal hydrophobic patch, EHP = external hydrophobic patch, Y = glycosylation sites. Fig.S2 Effects of MG132 on protein solubility. Soluble and insoluble fractions of cell lysates 48 hours after transfection with or without treatment of MG132 (5μM) were subjected to anti-uromodulin Western blotting. Fig. S3 Effect of lactacystine on the intracellular protein levels of WT and C112Y mutant. Representative immunoblots of mature, immature forms and secreted form of WT and C112Y mutant with or without treatment of lactacystine (25 μM). Cells were co-transfected with the WT or C112Y mutant and 2 EGFP plasmid. Cell lysates were prepared 48 hours after co-transfection and were subjected to Western blot with the indicated antibodies. Fig. S4 Effects of topiroxostat on the proteins level of intracellular and secreted WT and C112Y Representative immunoblots of mature, immature forms and secreted form of WT and C112Y mutant with or without treatment of topiroxostat (30 μM). Cells were co-transfected with the WT or C112Y mutant and EGFP plasmid. Cell lysates were prepared 48 hours after co-transfection and were subjected to Western blot with the indicated antibodies. The condition media were prepared 48 hours after co-transfection, concentrated and were subjected to Western blot with the indicated antibodies. 3