Membrane Air Separation Theory (RK)
advertisement

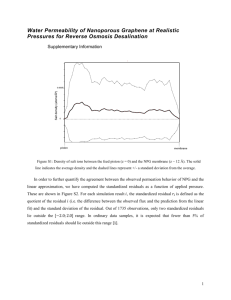
Membrane Air Separation Theory Microporous membranes perform essentially like sieves and allow small molecules to move through the pores while large molecules are blocked (Towler and Sinnot 562). Membrane separation works on the basis of relative permeation rates of the components being separated. Each component transporting through the membrane have a permeation rate that is unique and is a function of the ability to dissolve and diffuse. The main two relationships for this separation are Fick’s Law (1) which relates diffusion and Henry’s Law (3) which relates solubility. The permeate in this experiment is oxygen whereas nitrogen is the retentate where the main assumption is that there is only oxygen and nitrogen present in the gas. The first relation to show the diffusive flux through the membrane is expressed by Fick’s Law in equation 1 below. 𝐽𝑖 = 𝐷𝑖 𝐿 ∗ (𝐶𝑖𝑛1 − 𝐶𝑖𝑛2 ) (1) Where: Ji = Flux of component i in mol/m2s Di = Diffusivity of component i in m2/s L = Thickness of membrane in meters Cin1 = Concentration of component i inside membrane wall on feed side in mol/m3 Cin2 = Concentration of component i outside the membrane wall on permeate side in mol/m3 The total flux of component i, Ji, can then be calculated. 𝐽𝑖 = 𝑄𝑖𝑝 𝜌 𝑛𝐴 (2) Where: Qip = Volumetric flow rate of species i in the permeate in m3/s ρ =Density of permeate in mol/m3 A = Area of membrane, (in this case 2.7m2 per module) n = Number of modules used, (in this case 2) The from Henry’s Law. 𝐶𝑖𝑚 = 𝑆𝑖 𝑝𝑖 (3) Where: Cim = Concentration of component i inside the membrane wall in mol/m3 Si = Solubility constant for component i in the membrane in mol/m3Pa pi = Partial pressure of component i in the gas phase in Pa Permeation through the membrane is a function of diffusivity and solubility therefore: 𝑃𝑖 = 𝐷𝑖 𝑆𝑖 (4) Where: Pi = Component i membrane permeability in mol/m3sPa Di = Diffusivity of component i in m2/s Si = Solubility constant for component i in the membrane in mol/m3Pa Next the separation efficiency can be determined based off of different permeation rates of the gas components. 𝑃 𝛼𝑖𝑗 = 𝑃 𝑖 𝑗 (5) Where: αij = Separation factor Pi = Same as before An experimental separation factor is frequently used to quantify the separation of a binary system using mole fractions. ′ 𝛼𝑖𝑗 =( 𝑥𝑖𝑝 𝑥𝑗𝑝 𝑥𝑖𝑓 ) (6) 𝑥𝑗𝑓 ′′ 𝛼𝑖𝑗 =( 𝑥𝑖𝑝 𝑥𝑗𝑝 𝑥𝑖𝑓 ) (7) 𝑥𝑗𝑓 Where: α’ij =Separation factor based on the non-permeate composition α’’ij = Separation factor based on feed composition Next the recovery of oxygen and nitrogen can be determined. 𝑄𝑝 𝐶𝑂2𝑝 𝑂2 𝑅𝑒𝑐𝑜𝑣𝑒𝑟𝑦 = 𝑄 𝑓 𝐶𝑂2𝑓 𝑄 𝐶 𝑁2 𝑅𝑒𝑐𝑜𝑣𝑒𝑟𝑦 = 𝑄 𝑟 𝐶𝑁2𝑟 𝑓 𝑁2𝑓 (8) (9) Where: Qp = Volumetric flow rate of permeate in m3/s Qf = Volumetric flow rate of feed in m3/s CO2f = Molar concentration of oxygen in feed in mol/m3 CO2p = Molar concentration of oxygen in permeate in mol/m3 CN2f = Molar concentration of nitrogen in feed in mol/m3 CN2r = Molar concentration of oxygen in permeate in mol/m3 Finally the stage cut can be determined which is the fractional amount of the total feed entering the membrane that passes through as the permeate. 𝑆𝑇𝐴𝐺𝐸𝐶𝑈𝑇 = 𝑄 𝑄𝑝 𝑝 +𝑄𝑓 Where: Qp = Volumetric flow rate of permeate in m3/s Qf = Volumetric flow rate of feed in m3/s (10)