300715105054SusChemEAbstract_ConcentrationofGlycyrrhizicAcid
advertisement

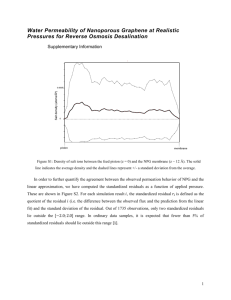
SusChemE 2015 International Conference on Sustainable Chemistry & Engineering October 8-9, 2015, Hotel Lalit, Mumbai Concentration of Glycyrrhizic Acid (GA) from Licorice Root Extract by Membrane Process Sandeep P Shewale1 , Virendra K. Rathod2 1, 2 Chemical Engineering Department, Institute of Chemical Technology, Matunga, Mumbai 400019, India *Corresponding author. Tel.: +91 22 33612020; fax: +91 22 33612010. E-mail addresses: sanshewale@gmail.com ,vk.rathod@ictmumbai.edu.in. 1. Introduction: Extraction gives much diluted amount of Glycyrrhizic Acid (GA) which further need to concentrate. Due to this limitation of extraction the membrane separation process used for concentration of GA. Effect of variation in different pressure was studied to monitor the permeate flux and rejection coefficient. Pressure has positive impact on the flux and rejection coefficient while the permeate concentration decreases with pressure. The feed, permeate and retentate were analysed and which shows that GA was more concentrated in retentate stream. 2. Material and Methods: 2.1 Material Licorice powder and HPLC grade water for the extraction. Analytical grade acetonitrile, acetic acid are the solvent and standard glycyrrhizic acid (GA) for the HPLC. PAN17 membrane for the concentration of GA and it was provided by CSIRNational Chemical Laboratory (NCL), Pune. 2.2 Methods The SS batch stirred cell with PAN17 membrane was used for experimentation which is shown in Fig 2. Pressures varied from 1, 1.5, 1.7 and 2.0 bars for different experimental run. By varying the parameters time, pressure experiments were performed and it was discussed for knowing the cross flow velocity, permeate flux, rejection coefficient. The known permeate was collected with time interval for analysis and also used for finding the flux [1]. Figure 1: Membrane Separation Experimental Setup . 3. Significant Results and Discussion 3.1 Membrane Performance The permeate flux was calculated for finding out the performance of membrane by using the following equation [2]. Permeate Flux (J) = Weight of Permeate Effective Area of Membrane X Time Rejection Coeffficient R i (%) = (1 − CP Cf ) X 100 (1) (2) Where Cp and Cf are concentrations of licorice solution in permeate and feed respectively 3.2 Effect of Pressure Over Membrane Results indicate that rejection was increased with increasing the pressure over the membrane and tends to be constant on the basis of concentration of feed and permeate by analytical method. The rejection coefficient was progressively augmented owing to the deposition of pure molecules. SusChemE 2015 International Conference on Sustainable Chemistry & Engineering October 8-9, 2015, Hotel Lalit, Mumbai 80 Permeate Flux (L/m2.h) 3.5 60 3 40 2.5 Mean Flux 2 Mean Rejection Coeff 1.5 20 Rejection Coefficieni in % 4 0 1.5 1.7 2 Pressure (Bar) Fig.3 Effect of pressure on mean permeates flux and mean rejection coefficient 3.3 Effect of Time on Permeate Concentration and Flux The permeate concentration was progressively decrease owing toward the deposition of natural molecules and configuration of a minor obstacle act as a film which prevent the way of other molecules throughout the membrane [3]. This result in lower concentration of species through the plane of membrane due to the scaling over the membrane will results the high concentration of GA in retentate i.e. higher rejection coefficient. 4. Conclusions Using membrane separation process with solvent extraction for the concentration GA form licorice root extract given good amount recovery in minimum energy and less time consumption. It can be alternative for the different conventional and costly techniques used for the concentrations of compounds from the extracted solution. References [1] T. W. Charpe, V.K. Rathod, Food and bioproducts processing. x x x, (2 0 1 3) xxx–xxx. [2] G. Justyna, M .N. Katarzyna, Environment Protection Engineering., 39 (3) 2013, 197-206. [3] M.R. Sohrabia, S.S. Madaenib,M. Khosravia, A. M Ghaedia, Separation and Purification Technology., 75 (2010) 121– 126 1