The Components of Water
advertisement
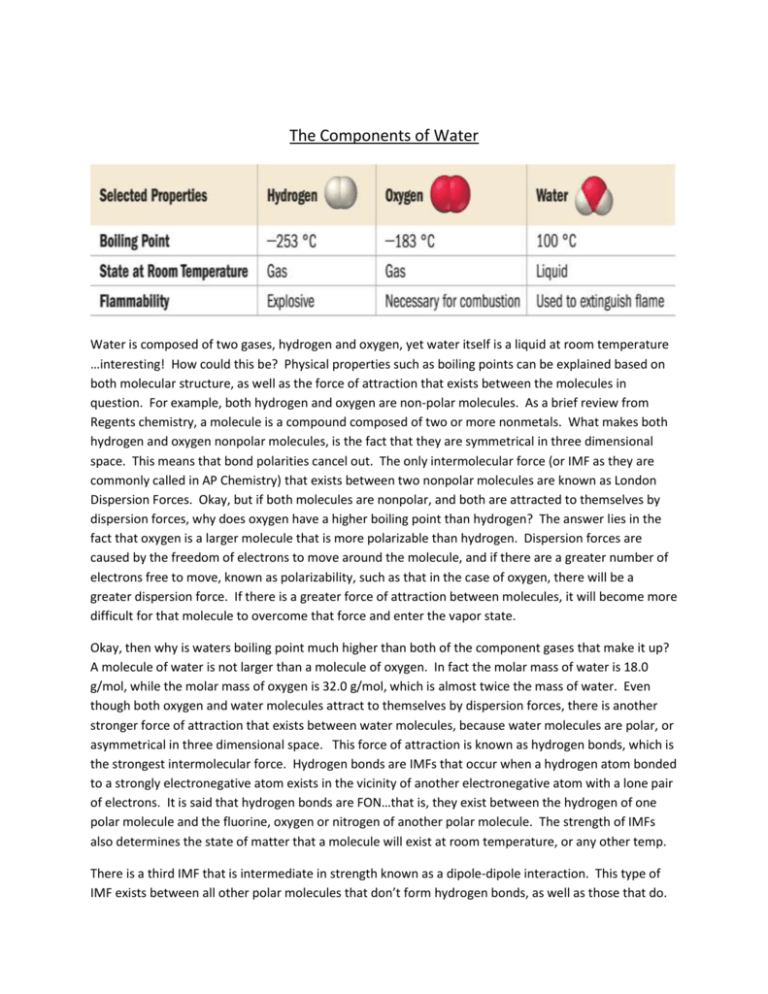
The Components of Water Water is composed of two gases, hydrogen and oxygen, yet water itself is a liquid at room temperature …interesting! How could this be? Physical properties such as boiling points can be explained based on both molecular structure, as well as the force of attraction that exists between the molecules in question. For example, both hydrogen and oxygen are non-polar molecules. As a brief review from Regents chemistry, a molecule is a compound composed of two or more nonmetals. What makes both hydrogen and oxygen nonpolar molecules, is the fact that they are symmetrical in three dimensional space. This means that bond polarities cancel out. The only intermolecular force (or IMF as they are commonly called in AP Chemistry) that exists between two nonpolar molecules are known as London Dispersion Forces. Okay, but if both molecules are nonpolar, and both are attracted to themselves by dispersion forces, why does oxygen have a higher boiling point than hydrogen? The answer lies in the fact that oxygen is a larger molecule that is more polarizable than hydrogen. Dispersion forces are caused by the freedom of electrons to move around the molecule, and if there are a greater number of electrons free to move, known as polarizability, such as that in the case of oxygen, there will be a greater dispersion force. If there is a greater force of attraction between molecules, it will become more difficult for that molecule to overcome that force and enter the vapor state. Okay, then why is waters boiling point much higher than both of the component gases that make it up? A molecule of water is not larger than a molecule of oxygen. In fact the molar mass of water is 18.0 g/mol, while the molar mass of oxygen is 32.0 g/mol, which is almost twice the mass of water. Even though both oxygen and water molecules attract to themselves by dispersion forces, there is another stronger force of attraction that exists between water molecules, because water molecules are polar, or asymmetrical in three dimensional space. This force of attraction is known as hydrogen bonds, which is the strongest intermolecular force. Hydrogen bonds are IMFs that occur when a hydrogen atom bonded to a strongly electronegative atom exists in the vicinity of another electronegative atom with a lone pair of electrons. It is said that hydrogen bonds are FON…that is, they exist between the hydrogen of one polar molecule and the fluorine, oxygen or nitrogen of another polar molecule. The strength of IMFs also determines the state of matter that a molecule will exist at room temperature, or any other temp. There is a third IMF that is intermediate in strength known as a dipole-dipole interaction. This type of IMF exists between all other polar molecules that don’t form hydrogen bonds, as well as those that do.











