Supporting Information
advertisement

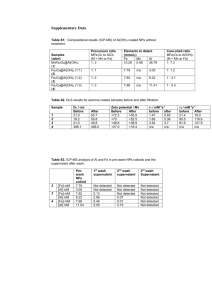
Supporting Information Synthesis, characterization and in vitro study of biocompatible Cinnamaldehyde functionalized magnetite nanoparticles (CPGF NPs) for hyperthermia and drug delivery applications in breast cancer Kirtee D. Wani, MSc1, Brijesh S. Kadu, MSc2, Prakash Mansara, MSc1, Preeti Gupta, MSc3, Avinash V. Deore, MSc4, Rajeev C. Chikate, PhD2, Pankaj Poddar PhD3, Sanjay D. Dhole, PhD4, Ruchika Kaul-Ghanekar, PhD*1 1 Cell and Translational Research Laboratory, Interactive Research School for Health Affairs (IRSHA), Bharati Vidyapeeth University Medical College Campus, Dhankawadi, Pune, Maharashtra, India. 2 Nanoscience group, Department of Chemistry, Post-graduate & Research Center, MES Abasaheb Garware College, Pune, Maharashtra, India. 3 Physical & Material Chemistry Division, CSIR-National Chemical Laboratory, Pune, Maharashtra, India. 4 Department of Physics, University of Pune, Pune, Maharashtra, India. File S1. Cytotoxic studies of cinnamaldehyde and its derivatives (Table S1) as well as those of MNPs loaded with herbal active compounds (Table S2). Physical characterization of F, G, C, P, GF, PGF and CPGF NPs (Figures S1-S4) and cytotoxicity data of F, G and P (Figure S5). Table S1. Cytotoxic studies of cinnamaldehyde and its derivatives Cancer Cell Lines Cinnamaldehyde type K562 transcinnamaldehyde 2′-benzoyloxy cinnamaldehyde MCF-7 MDA-MB-231 MDA-MB-435 A375 G361 LOX HT29 HCT116 Hs27 HEK 293 HT1080 A431 SK-N-MC MG63 Neuro2a SiHa L929 Raw264.7 Mouse primary fibroblast Mouse Hepatocyte MCF10A HEK293 MDAMB231 MCF7 MDAMB231 MCF7 Cinnamaldehyde Cinnamaldehyde Cinnamaldehyde CPGF NPs containing Cinnamaldehyde Concentration range used (μM) 120 and 180 15-60 1-25 Not given Not given Not given Not given Not given Not given 0.8-320 5-160 1.75-900 ×10-3 MIC (μM) IC50 (μM) References Not given 15 ------ Zhang et al., 2010 Ismail et al., 2012 5 ------------------40 40 10 160 40 20 20 80 3.2 57.2 27.7 24.5 6.3 8.1 3.4 19.7 12.6 21.4 17.9 ------------------------------------- 5 ----- 80 80 40 80 0.014 334.8 184.4 69.81 284.7 0.363 0.112 0.368 Cabello et al., 2009 Singh et al., 2009 (Our previous work) Present work submitted to PLOS One References Zhang JH, Liu LQ, He YL, Kong WJ, Huang SA (2010) Cytotoxic effect of transcinnamaldehyde on human leukemia K562 Cells. Acta Pharmacol Sin 31:861-866. Ismail IA, Kang HS, Lee HJ, Kwon BM, Hong SH (2012) 2'- Benzoyloxycinnamaldehyde-mediated DJ-1 upregulation protects MCF-7 cells from mitochondrial damage. Biol Pharm Bull 35:895-902. Cabello CM, Bair WB 3rd, Lamore SD, Ley S, Bause AS, et al. (2009) The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic Biol Med 46:220-231. Singh R, Koppikar SJ, Paul P, Gilda S, Paradkar AR, et al. (2009) Comparative analysis of cytotoxic effect of aqueous cinnamon extract from Cinnamomum zeylanicum bark with commercial cinnamaldehyde on various cell lines. Phar Bio 47:1174-1179. Table S2. Cytotoxic studies of MNPs loaded with herbal active compounds Herbal active compounds loaded onto MNPs-Fe3O4 Artesunate Cancer Cell Type Concentration Minimum IC50 References (μM) inhibitory (μM) concentration K562 12.5-100 12.5 µM Silibinin T47D 20 µM Gambogic acid Capan-1 20-120 µM 20-120 µM 0.25-2.0 µM Wogonin Raji cells 12.5-150 µM 12.5 µM Curcumin A2780CP 5–40 μM MDAMB231 PC3 MCF7 0.47-15 µg/mL 5 μM 5 μM 5 μM 7 µg/mL HT29 7 µg/mL Gallic acid 10-100 μM Genistein SGC-7901 Cinnamaldehyde MDAMB231 1.75-900 nM 0.25 µM 10 μM 0.014 μM Not Wang et al., given 2011 73 Ebrahimnezhad et al., 2013 Not Wang et al., given 2011 80 Ren et al., 2012 12.1 11.9 12.8 Not given Not given Not given 0.363 Yallapu et al., 2011 Dorniani et al., 2012 Si et al., 2010 Present work (in CPGF NPs) 0.112 μM MCF7 0.368 submitted to PLOS One References Wang Y, Han Y, Yang Y, Yang J, Guo X, et al. (2011) Effect of interaction of magnetic nanoparticles of Fe3O4 and artesunate on apoptosis of K562 cells. Int J Nanomedicine 6:1185–1192. Ebrahimnezhad Z, Zarghami N, Keyhani M, Amirsaadat S, Akbarzadeh A, et al. (2013) Inhibition of hTERT gene expression by silibinin-loaded PLGA-PEG-Fe3O4 in T47D breast cancer cell line. Bioimpacts 3:67-74. Wang C, Zhang H, Chen B, Yin H, Wang the enhanced anticancer efficacy of gambogic cancer cells when mediated via magnetic W (2011) Study of acid on Capan-1 pancreatic Fe3O4 nanoparticles. Int J Nanomedicine 6:1929–1935. Ren Y, Zhang H, Chen B, Cheng J, Cai X, et al. (2012) Multifunctional magnetic Fe3O4 nanoparticles combined with chemotherapy and hyperthermia to overcome multidrug resistance. Int J Nanomedicine 7:2261-2269. Yallapu MM, Othman SF, Curtis ET, Bauer NA, Chauhan N, et al. (2012) Curcuminloaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomedicine 7:1761–1779. Dorniani D, Hussein MZB, Kura AU, Fakurazi S, Shaari AH, et al. (2012) Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int J Nanomedicine 7:5745–5756. Si HY, Li DP, Wang TM, Zhang HL, Ren FY, et al. (2010) Improving the anti-tumor effect of genistein with a biocompatible superparamagnetic drug delivery system. J Nanosci Nanotechnol 10:2325–2331. Figure S1. TEM images of F, GF and PGF NPs TEM image showed the particle size to be around 5-10 nm for F and GF NPs and ~10-15 nm for PGF NPs. This indicated that the size of the NPs gradually increased from ~5 nm for nonconjugated Fe3O4 to ~20 nm for PGF NPs by successive layering of G and P onto the F NPs. F GF PGF Figure S1. TEM images of F, GF and PGF NPs Figure S2. FTIR spectra of F, G and GF Glycine spectrum (G) showed remarkable signatures at ~3200 cm–1 for -NH2 or -OH and that for carboxylate stretch at ~1600 cm–1. The notable difference between Fe3O4 spectrum (F) and glycine capped Fe3O4 (GF), was the appearance of a strong new signature at 3200 cm–1, along with 1600 cm–1. This indicated that Fe3O4 surface was efficiently capped with glycine. Figure S2. FTIR spectra of F, G and GF Figure S3. FTIR spectra of GF, P and PGF The appearance of peaks at 3200 cm–1 and 1600 cm–1 (1696 cm–1 in PGF) for both GF and PGF suggested that the hydroxyl/amine and carboxylate functions of G and P are successively loaded onto the Fe3O4 NPs. The overall merging of peaks indicated that pluronic possessed similar functional groups as in glycine resulting in similar spectrum of PGF to GF. Figure S3. FTIR spectra of GF, P and PGF Figure S4. FTIR spectra of PGF, C and CPGF Cinnamaldehyde spectrum (C) showed prominent peaks at 1696, 1634, 1140, 960 and 764 cm–1 arising due to bonds specified by aldehyde structure. There was an interesting shift of –C=C– and –C=O stretches from 1696 and 1634 cm–1 in PGF towards higher energy side at 1709 and 1695 cm–1 in CPGF denoting conjugation of cinnamaldehyde with PGF. The invariable presence of other peaks in cinnamaldehyde such as 1140, 960 and 764 cm–1 in CPGF spectrum suggested that cinnamaldehyde was electrostatically bound to PGF. Figure S4. FTIR spectra of PGF, C and CPGF Figure S5. Cytotoxicity of F, G and P The effect of F, G and P on MDAMB231 and MCF7 was analyzed by using MTT dye. The cells were treated with 0-640 µg/ml of F, G and P. Uncoated Fe3O4 nanoparticles were nontoxic to all the cell lines which is in accordance with the previously reported data. Both the cell lines showed ≥100% viability post-treatment with glycine and pluronic used to coat Fe3O4 nanoparticles. Therefore, these coating materials were non-toxic and safe. Figure S5. Cytotoxicity of F, G and P on breast cancer cell lines