Chemical Formulas_PPT
advertisement

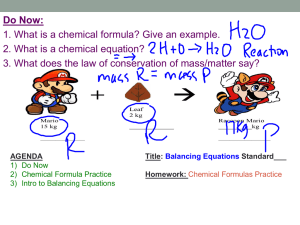
Chemical Formulas Chemical Formula: An easy way for scientist to describe a molecule using the element symbols. H 2O C02 REMEMBER… When you read a chemical formula, each new element begins with a capital letter. So if you see CO2, you should think Carbon and Oxygen. However… If you see a lower case letter in a chemical formula, it goes with the capital letter that comes before it. For example: If you see NaCl, you should think (Na)Sodium and (Cl)Chlorine. Reading Chemical Formulas NaCl Sodium Chlorine Reading Chemical Formulas This subscript tells us there are 2 atoms of hydrogen in this formula. H2O Hydrogen If no subscript is present, it is understood the subscript is 1. So there is 1 Atom of oxygen in this formula Oxygen Subscript – number that follows a symbol; subscript tells how many atoms of element are in molecule Reading Chemical Formulas The coefficient shows us there are 2 molecules of water in this formula. 2H2O Hydrogen Coefficient: The coefficient in this formula tells us there are 4 hydrogen atoms and 2 oxygen atoms Oxygen The large number before an element symbol or compound that represents the number of elements or compounds. You multiply the coefficient by the subscript to find the number of atoms of each element. Reading Chemical Formulas 2CO2 Carbon Oxygen What elements are bonded to create this molecule of Carbon Dioxide? How many atoms of each element are in this molecule of Carbon Dioxide?