1 H NMR Metabolomics Study of Metastatic
advertisement

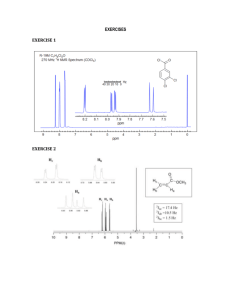
Supporting information 1 H NMR Metabolomics Study of Metastatic Melanoma in C57BL/6J Mouse Spleen Xuan Wang1, 2, Mary Hu1, Ju Feng1, Maili Liu2, Jian Zhi Hu1* (1) Pacific Northwest National Laboratory, Richland, WA 99352, USA (2) Wuhan Institute of Physics and Mathematics, the Chinese Academy of Sciences, Wuhan, 430071, PR China. * To whom correspondence should be addressed: Jian Zhi Hu; Email: Jianzhi.Hu@pnnl.gov; Phone: (509) 371-6544; Fax: (509) 371-6546 1 Fig. S1 Light microscopy images (20X) of spleen tissue from the tumor cell treated (left) and the control (right) mice. Melanocarcinoma masses were characterized by the abnormal size, shape and morphology of the nuclei Fig. S2 PCA scores plots of spleen tissue extracts from the control (blue dots) and tumor (green dots) groups: (a), data derived from binning results of 1H NMR spectra of hydrophilic extracts; (b), metabolites concentrations obtained by spectral deconvolution of hydrophilic extracts; (c), data derived from binning results of 1H NMR spectra of hydrophobic extracts 2 Table S1 Solvents-Tissue ratio for extraction MeOH H2O CHCl3 CHCl3 H2O (ml) (μl) (ml) (ml) (ml) 1g 4 850 2 2 2 0.5 g 2 425 1 1 1 0.25 g 1 213 0.5 0.5 0.5 125 mg 0.5 106 0.25 0.25 0.25 62.5 mg 0.25 53 0.125 0.125 0.125 31.3 mg 0.25 53 0.125 0.125 0.125 10.0 mg 0.25 53 0.125 0.125 0.125 Tissue Table S2 Correlation coefficients of all metabolites used for OPLS analysis Key Metabolites Correlation coefficients 1 Isoleucine 0.563 2 Alloisoleucine 0.370 3 Leucine 0.736 4 2-Aminobutyrate 0.670 5 Valine 0.536 6 Isobutyrate 0.579 7 3-Hydroxyisobutyrate 0.197 8 Ethanol 0.065 9 3-Hydroxybutyrate -0.274 10 Fucose 0.154 11 Lactate 0.404 12 Threonine 0.643 13 Lysine 0.604 3 14 Alanine 0.817 15 Arginine 0.233 16 Thymidine 0.832 17 Acetate 0.122 18 dTTP 0.311 19 Glutamate -0.911 20 Glutamine 0.282 21 Methionine 0.514 22 Glutathione 0.743 23 dCTP 0.874 24 Malate 0.897 25 2-Oxoglutarate 0.564 26 Isocitrate -0.504 27 2'-Deoxyguanosine 0.854 28 Citrate 0.617 29 Aspartate -0.870 30 Asparagine 0.771 31 Trimethylamine 0.679 32 Tyramine 0.323 33 Histamine 0.936 34 Creatine phosphate 0.596 35 Creatinine -0.554 36 Creatine -0.581 37 Tyrosine 0.462 38 Phenylalanine 0.616 39 Histidine 0.585 40 Ethanolamine 0.275 41 Choline 0.430 4 42 O-Phosphocholine 0.248 43 O-Phosphoethanolamine -0.967 44 sn-Glycero-3-phosphocholine -0.020 45 π-Methylhistidine 0.773 46 Trimethylamine N-oxide 0.185 47 Glucose 0.168 48 Taurine -0.892 49 Betaine 0.498 50 myo-Inositol -0.076 51 Tryptophan 0.501 53 UDP-glucuronate 0.132 54 Glycine 0.772 55 Glycerol -0.252 56 UDP-galactose 0.149 57 Uridine 0.232 58 Cytidine -0.240 59 Adenosine 0.383 60 Inosine -0.186 61 Serine 0.606 62 AMP 0.775 63 ATP -0.936 64 ADP -0.107 65 GTP 0.924 66 Uracil 0.621 67 Fumarate 0.611 68 Benzoate -0.138 69 Niacinamide -0.925 70 Xanthine 0.931 5 71 Hypoxanthine 0.012 72 Oxypurinol -0.046 73 Formate -0.433 * Unsigned (δ7.68) 0.562 Broadline filtering strategy for spectral deconvolution Currently, there are no commonly accepted methods for treating the broadline features in a high resolution 1H NMR metabolite spectrum. Many researchers use baseline correction methods to filter out the broadline features before performing spectral deconvolution of metabolites that have narrow line widths. The shortcoming of baseline correction is that it heavily depends on the skill of the individual who conducts the fitting and the method is not reproducible. This will affect the quantitation of the metabolites, in particular in cases where metabolites with low intensity spectral peaks are of high importance. To better fit the spectra, in this work a new strategy is proposed, where broad peaks corresponding to lipoprotein signals and compounds exhibiting slow reorientation such as high density lipoprotein (HDL) and low density lipoprotein (LDL), are first built in the Compound Builder module of Chenomx using the peak-based method introduced in the software tutorial. The compound signatures for the HDL and LDL are based on published literatures (Coen et al. 2003; Lindon et al. 1999; Nicholson et al. 1995). These customized broad peaks are then added to the Library Manager module of Chenomx as new compounds so that they can be used to construct the broaline features associated with a high resolution liquid state 1H NMR metabolite spectrum. Firstly, the broadline feature in a selected spectrum from one sample type of one tissue type is constructed by utilizing a group of customized spectral peaks and by adjusting both the intensities and the linewidths of the customized spectral peaks so that the overall envelop fits the envelop of the broadline features in the experimental spectrum. Once the broaline feature spectrum associated with a particular sample type and tissue type is built, it can be effectively utilized to fit other spectra of the tissue type in a series of experiments with only minor adjusting of the intensities of the customized peaks. This new strategy in addressing the broadline features takes the advantages of Chenomx where optimization of peak positions and intensities is realized using the Compound 6 Builder module of Chenomx, resulting in high flexibility and reproducibility. The method is demonstrated in Figure S3, where a typical 1H NMR spectrum of hydrophilic extracts from δ 0.5 to δ 1.15 is shown. Figure S3 (a) shows individual broadline features (blue) that have been constructed using the Compound Builder module of Chenomx in the corresponding spectral region. Figure S3 (b) shows the best overall fitting quality, demonstrating that the experimental spectrum (black) is well deconvoluted as is evident by the difference spectrum (green). An overfit will show a negative residual peak (green) while an underfit will leave a positive residual peak in the difference spectrum (green). The red line, i.e., the summed spectrum, displays the combined shape of all the profiled metabolites. Deconvolution of the other spectral regions is conducted using the same strategy demonstrated in Figure S3 and the overall fitting quality of an entire experimental spectrum is illustrated in Figure S4. As shown in the figure, the original spectrum is well fitted except for the unfitted residual water peak. Once the first overall model for the broadline feature spectrum is constructed and saved in the Compound Builder module of Chenomx, all the remaining 9 spectra from both the control and the tumor groups can be easily fit by calling out the model and by optimizing the intensities of the individual broad peaks. In this way, all the ten spectra of hydrophilic extracts from both the control and the tumor groups are fitted with consistent strategies for handling broadline features and with reproducible fitting results. 7 Fig. S3 Illustration of the deconvolution strategy: (a), individual broadline features (blue) in the corresponding spectral region constructed using the Compound Builder module of Chenomx: 1, albumin; 2, HDL-CH3; 3, VLDL/LDLCH3; 4, lipids-CH3CH2; 5, a broadline feature; (b), the overall fitting quality of the spectral region: experimental spectrum (black), best fitted spectrum (red), fitting error/difference spectrum (green) 8 Fig. S4 A representative 1H NMR Spectrum of hydrophilic extracts from the tumor group. The bottom trace spectrum is the entire spectrum ranged from -0.5 ppm to 9.5 ppm accompanied by the upper three traces of expanded regions giving the detailed fitting results 9 Reference Coen, M., Lenz, E. M., Nicholson, J. K., Wilson, I. D., Pognan, F., & Lindon, J. C. (2003). An integrated metabonomic investigation of acetaminophen toxicity in the mouse using NMR spectroscopy. Chemical Research in Toxicology, 16(3), 295-303. Lindon, J. C., Nicholson, J. K., & Everett, J. R. (1999). NMR spectroscopy of biofluids. Annual Reports on Nmr Spectroscopy, Vol 38, 38, 1-88. Nicholson, J. K., Foxall, P. J. D., Spraul, M., Farrant, R. D., & Lindon, J. C. (1995). 750-Mhz H-1 and H-1-C-13 Nmr-Spectroscopy of Human Blood-Plasma. Analytical Chemistry, 67(5), 793-811. 10