Test H - Science @ St John`s
advertisement

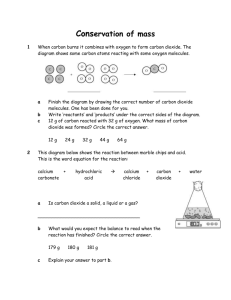
C1 Knowledge Test 1. 2. 3. 4. 5. 6. Write the formula for the first three alkanes Write the formula for the first two alkenes How do we test for the presence of alkenes? What process breaks down large hydrocarbons? What substance is formed from the polymerisation of ethane? Complete this table to give the uses of these polymers: Polymer Polyethene Polypropene Polychloroethene (PVC) Teflon 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. What mineral is limestone made from? Give two disadvantages of quarrying limestone Give one advantage of quarrying limestone Chalk, limestone and marble are all made of which mineral? Calcium carbonate is mined to form which three everyday items? Write a word equation for the thermal decomposition of limestone Where did the gases that formed the Earths early atmosphere come from? How do scientists use rocks to work out the composition of the Earth’s early atmosphere? Name 4 gases other than oxygen and carbon dioxide that were present in the Earth’s early atmosphere. How much CO2 was present in the Earths early atmosphere? Describe two ways that the carbon dioxide levels in the early atmosphere were reduced. How much oxygen was present in the Earths early atmosphere? How did photosynthesis affect the composition of the atmosphere? How do scientists believe the oceans were formed? Complete this table to show the current composition of the Earths atmosphere: Gas 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. Formula % in dry air What effect does deforestation have on the amount of carbon dioxide in the atmosphere. Explain your answer State 3 ways humans changed the composition of the atmosphere. How do we test for carbon dioxide? What two different substances does incomplete combustion produce as compared to complete combustion? Give two ways scientists are trying to reduce the amount of carbon dioxide in the atmosphere. Why is carbon monoxide toxic? What 2 main gases do volcanoes release into the atmosphere. What type of rock is marble? Name 2 sedimentary rocks How are sedimentary rocks formed? What two factors are required to form metamorphic rock? What are igneous rocks formed from? What affects the size of crystals in in igneous rock? 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. Name an igneous rock Which type of rock may contain fossils? Which type of rock is easy to wear away? What can you say about the mass of reactants and the mass of products in a reaction? What are the smallest particles of an element that can take part in a reaction? Write a word equation for the reaction between water and calcium oxide. What is formed when calcium hydroxide is dissolved in water? Give 2 uses of calcium carbonate in industry. Give 2 uses of hydrochloric acid in the stomach. Write a word equation for the reaction between hydrochloric acid and calcium carbonate What type of salts are formed from hydrochloric acid; nitric acid; sulfuric acid How do we test for oxygen? How do we test for Hydrogen? How do we test for chlorine? What three substances can neutralise acids? What process decomposes compounds using an electrical current? How can chlorine be extracted from seawater? Name two substances produced from chlorine. What is produced during the electrolysis of water? Name the top two and bottom two metals in the reactivity series Why is gold used for jewellery? Why is copper used for electrical wires? Where do most metals originate? What is an alloy? Why is iron often alloyed? State two uses of new alloys developed by scientists. Describe two ways of extracting metals from their ores. What type of reaction involves gain of oxygen? What is another term for the oxidation of metals? Which part of the reactivity series contains metals least likely to corrode? What two elements do hydrocarbons contain? Write a word equation for the combustion of a hydrocarbon. Crude oil is a mixture of… What is the purpose of fractional distillation? Complete this table to give a use of each of these fractions: Fraction Gases petrol Kerosene Diesel oil Fuel oil Bitumen 71. 72. 73. 74. 75. 76. Use What happens to the boiling point of the fractions as they get longer? What gas is produced due to the impurities in fossil fuels? What substance weathers limestone, damages trees and makes lakes and rivers acidic? Name 3 greenhouse gases. What is ethanol produced from? Give 4 factors of a good fuel. C1 Knowledge Test Answers 1. CH4, C2H6, C3H8 2. C2H4, C3H6 3. Bromine water will discolour 4. Cracking 5. Polyethene 6. . Polymer Polyethene Polypropene Polychloroethene (PVC) Teflon 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. Use Plastic bags, plastic bottles, cling film, insulation for electrical wires Buckets and bowls Window frames, gutters, pipes, insulation for electrical wires Non stick coatings for saucepans, bearings for skis, containers for corrosive substances, stain proof coating for carpets, insulation for electrical wires Calcium Carbonate Noise pollution, destroys habitats, land cannot be used for farming, destroy views Jobs, valuable export. Calcium Carbonate Glass, cement and concrete Calcium carbonate Calcium oxide + carbon dioxide Volcanic activity Analyse the minerals in them and look for oxides. Nitrogen, ammonia, methane, water vapour Lots CO2 dissolved into the oceans, incorporation into marine organisms shells. Little/none Removed CO2, added O2 Condensation of water vapour. . Gas Formula % in dry air Nitrogen N2 78 Oxygen O2 21 Argon Ar 0.9 Carbon Dioxide CO2 0.04 other trace 22. Increases it 23. Trees no longer remove carbon dioxide through photosynthesis. 24. Burning fossil fuels, farming, deforestation 25. Turns limewater cloudy 26. Carbon monoxide and carbon 27. Iron seeding, converting carbon dioxide into hydrocarbons 28. Takes the place of oxygen in RBC 29. Carbon dioxide and nitrogen 30. Metamorphic 31. Chalk and limestone 32. Compaction of layers of sediment over a long time 33. Heat and pressure 34. Cooled magma or lava 35. The rate at which it cooled down 36. Granite 37. Sedimentary 38. Sedimentary 39. The same 40. Atoms 41. Calcium oxide + water Calcium hydroxide 42. Limewater 43. Neutralise soils, remove gases from powerstations 44. Helps digestion, kills bacteria 45. hydrochloric acid + calcium carbonate calcium chloride + water + carbon dioxide 46. Chlorides, nitrates, sulfates 47. Relight a glowing splint 48. Burns with a squeaks pop 49. Bleach damp blue litmus paper 50. Metal oxides, metal hydroxides, metal carbonates 51. Electrolysis 52. Electrolysis 53. Bleach, PVC 54. Hydrogen and oxygen 55. Potassium, sodium and gold, platinum 56. Unreactive and does not corrode 57. Good electrical conductor 58. In the Earths crust as ores 59. A mixture of two metals 60. Increase strength and reduce corrosion 61. Stents in damaged blood vessels, memory spectacle frames 62. Heating/reduction with carbon, electrolysis 63. Oxidation 64. Corrosion 65. Bottom 66. Hydrogen and carbon 67. Hydrocarbon + Oxygen Carbon Dioxide + Water 68. Hydrocarbons 69. Splits crude oil into simpler substances 70. . Fraction Use Gases Domestic heating and cooking petrol Fuel for cars Kerosene Fuel for aircraft Diesel oil Fuel for cars and trains Fuel oil Fuel for ships and power stations Bitumen Surfacing roads and roofs 71. Increases 72. Sulfur dioxide 73. Acid rain 74. Carbon dioxide, methane, water vapour 75. Sugar beet or sugar cane 76. Ease of burning, ease of transport, amount of smoke produced, amount of heat energy liberated.