Reading Chemical Formulas: Element
advertisement

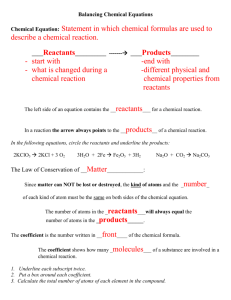
Reading Chemical Formulas: Element- made up of only one kind of atom. The simplest form of matter. Molecule- when two or more atoms combine (Example: O2) Compound- when two or more different atoms combine (Example: CO2) Every element on the periodic table is represented by a symbol. Molecules and compounds are represented by a combination of the element’s symbol and numbers representing the number of atoms or molecules in the compound. For example, H2 is the chemical formula for a molecule of Hydrogen. The H is the chemical symbol for hydrogen and the subscript 2 indicates that there are 2 atoms of hydrogen in that molecule. H2O is the compound for water representing two atoms of Hydrogen and one of Oxygen! Coefficient- The number in front of the element or compound. It is equal to the number of molecules! Subscript- The number after and below the element symbol. It is equal to the number of atoms of that element present in the compound! **If there is NO coefficient or subscript it is understood to be 1** A chemical equation is a way of describing a chemical reaction using chemical formulas. Ex: Hydrogen and Oxygen atoms react chemically to form water. The chemical equation that shows this is H2 + O ***We call the molecules on the left side of the equation are always called the REACTANTS. They “react” to form the product. The molecules on the right side of the equation are always called the PRODUCTS*** Law of conservation of mass- states that matter cannot be created or destroyed. This means that the number and kind of atoms in the reactants must equal the number and kinds of atoms in the product! If that is true then we say the equation is balanced! PRACTICE: C6H12O6 Has 3 elements: Carbon (C), Hydrogen (H), and Oxygen (O) Has 6 atoms of carbon, 12 atoms of Hydrogen, and 6 atoms of oxygen 2H2O Has 2 molecules of water. So to find the number of atoms of each element we have the distribute the coefficient. That means multiply the coefficient by the subscript. 2 x 2 = 4 so there are 4 atoms of Hydrogen 2 x 1 = 2 remember if there is no subscript we assume it is one so there are 2 atoms of oxygen Chemical Reactions In a chemical reaction elements are rearranged to create new substances with different properties :) Don't forget that these equations must be balanced (have the same number of atoms of each element on both sides) because the Law of Conservation of Mass says that matter cannot be created or destroyed. Evidence that a CHEMICAL CHANGE has occurred: That means if you see any of the things below a chemical change NOT a physical change has occurred: Please- Precipitate (a solid that forms from a solution) Excuse- Endothermic (heat is absorbs, it feels cold)/ Exothermic (heat is released it feels hot) Coughs-Color change (unexpected not from something like food coloring) Sneezes-Smell (A new smell is produced) Burps-Bubbles (a gas is being produced) Or-Oxidation (rust) Chunks-Combustion (fire)