Freezing Point Depression Lab
advertisement

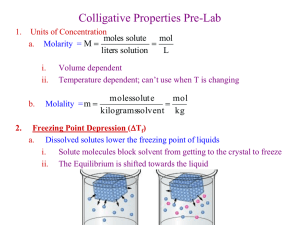
Freezing Point Depression Lab Goal: Determine the effect of dissolved impurities on the freezing point of water and determine which impurities have the greatest effect. Chemical Concepts: Freezing/Melting Freezing Point Depression Colligative properties Van’t Hoff Factor (Dissociation of ions) Background: Inhabitants of northern states or in mountainous regions are familiar with winter and the snowy, icy roads that result. Road crews spread salt (sodium chloride, calcium chloride, or a variety of salt mixtures) on the road in order to lower the temperature at which freezing occurs. Thus, the surface of the treated road does not freeze until the temperature gets much lower than 0oC. If the road already has ice on it, the salt helps to melt the ice, forming a solution with a lower freezing point than that of pure water. During the freezing process of water, for example, water molecules come together to form the more orderly, crystalline pattern of ice molecules. When any solute (such as salt) is added to a solvent (such as water), the pattern is interrupted by the presence of the salt “impurity”. These impurities interfere with the ability of the solvent to crystallize (solidify) and the solution remains liquid even at a lower temperature. Thus a solution always has a lower freezing point than its pure solvent. This phenomenon is termed freezing point depression. Freezing point depression (Tf) is defined as the difference in temperature between the freezing point of a solution and that of its pure solvent. The freezing point of the solution after the addition of a solute can be calculated by the following equation: Tf = Kf x m x i Here, Tf is the change in freezing point (the freezing point depression) in oC, Kf is the freezing point depression constant (1.86 oC/m for water solutions), m is the molality of the solution (the solution concentration in moles of solute per kilogram of water), and i is the number of ions (particles, Van’t Hoff factor) formed from the dissociation of the solute into individual ions when added to solvent. Materials: Aluminum chloride hexahydrate Calcium chloride dihydrate Sodium Chloride Sucrose Ice Water-water with crushed ice chunks Balance 4 small styrofoam or paper cups LabQuest with temperature probe or thermometer Wooden stirrers or glass stirring rod 100 ml beaker to weigh out chemicals Safety and Disposal Considerations: Aluminum chloride and calcium chloride are slightly toxic by ingestion. So don’t eat it. Aluminum chloride solution must be collected in waste container. The other solutions can be dumped down the sink. Procedure: 1. Label four cups with numbers 1-4. 2. Place cup #1 on balance and zero (tare). 3. Make sure your partner saves your tare while you take cup #1 to cooler and scoop up about 20 grams of ice water. Instructor will give you a guideline as to the approximate amount this is. Note: It is important that you have both water and ice in the cup. 4. Stir the ice water with a glass stirring rod or wooden stirrer. 5. Carefully insert thermometer or temperature probe into cup #1. Continue to stir the mixture (not with thermometer or probe) while watching temperature lower. Record in the data table the lowest temperature that the mixture reaches before rising again. Record the temperature of the pure ice water mixture in oC to the nearest tenth of a degree. Cup #1 – Sucrose 6. In a 100 mL beaker, weigh out 17.56 g of sucrose (does not have to be exact, just close). Record your exact mass in data table. 7. Add the sucrose to the ice water mixture in cup #1. 8. Stir the contents of the cup until the mixture has a slushy appearance. 9. Carefully insert thermometer or temperature probe into cup #1. Continue to stir the mixture (use stirring rod not thermometer!) while watching temperature lower. Record in the data table the lowest temperature that the mixture reaches before rising again. This might take some time, as the solute does not dissolve immediately, so it is a good idea to have your partner work on the next sample. Cup #2 – Sodium Chloride 10. Repeat steps 2-9 (skip steps 4 and 5) for cup #2 using about 20 grams of ice water and 3.0 g of NaCl. Cup #3 – Calcium Chloride 11. Repeat steps 2-9 (skip steps 4 and 5) for cup #3 using about 20 grams of ice water and 7.54 g of CaCl2•2H2O. Cup #4 – Aluminum Chloride 12. Repeat steps 2-9 (skip steps 4 and 5) for cup #4 using about 20 grams of ice water and 6.19 g of AlCl3•6H2O. Reporting Data to Class 13. Place your lowest temperature values for each sample on the white board as instructed by teacher. 14. Record the class average of lowest temperature for each sample before class is over in the space provided on your data table.