Drug Dosage Calculations (No Accomodations)
advertisement
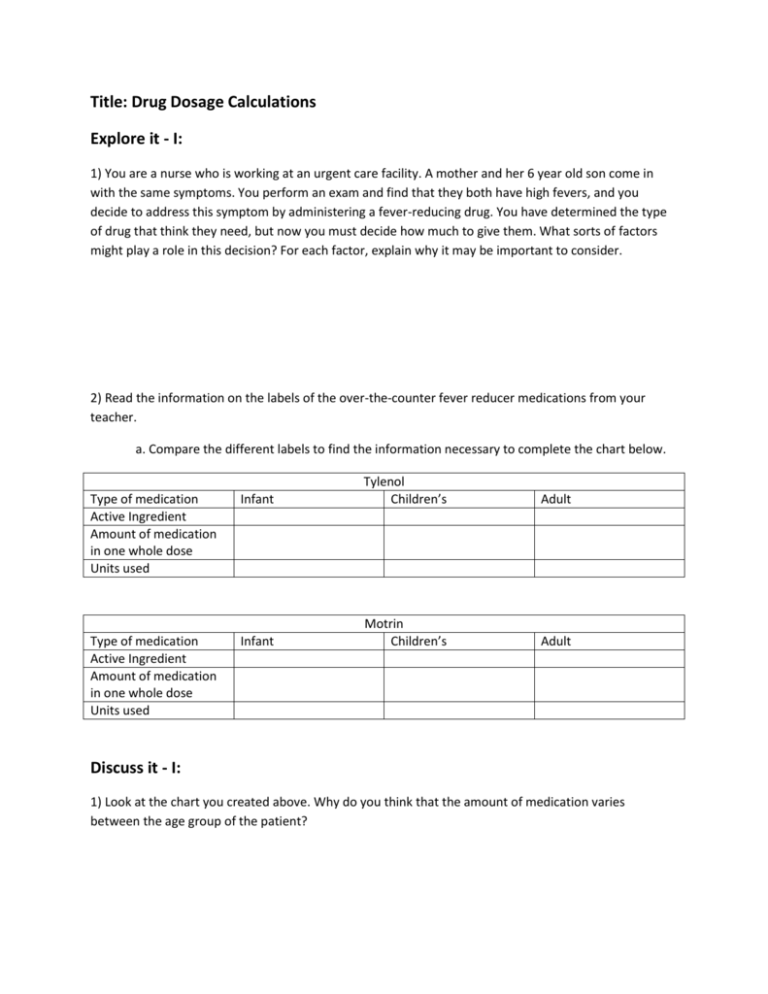
Title: Drug Dosage Calculations Explore it - I: 1) You are a nurse who is working at an urgent care facility. A mother and her 6 year old son come in with the same symptoms. You perform an exam and find that they both have high fevers, and you decide to address this symptom by administering a fever-reducing drug. You have determined the type of drug that think they need, but now you must decide how much to give them. What sorts of factors might play a role in this decision? For each factor, explain why it may be important to consider. 2) Read the information on the labels of the over-the-counter fever reducer medications from your teacher. a. Compare the different labels to find the information necessary to complete the chart below. Type of medication Active Ingredient Amount of medication in one whole dose Units used Infant Tylenol Children’s Type of medication Active Ingredient Amount of medication in one whole dose Units used Infant Motrin Children’s Adult Adult Discuss it - I: 1) Look at the chart you created above. Why do you think that the amount of medication varies between the age group of the patient? 2) Look at the chart you created. Within one age group, compare the different types of fever-reducing drugs. Why do you think the amount of medication varies between the different drug types? 3) What are some of the warnings described for the different drugs that are related to dose? 4) How might the dosing instructions and the warnings be related? 5) What do you think is meant by ‘active ingredient?’ Explore it II: You learned in part 1 that doses will differ from person to person and from drug to drug. For each drug, you also learned that when someone takes a drug, the medication they need is mixed with other ingredients. So how much of the pill actually contains medicine? One way to mathematically represent how much of the dose of a drug is made up of the active ingredient is by describing it as a percent. 1) What information would you need to know about one dose of medicine to be able to calculate the percent of the dose that is made up of the active ingredient? How would you use that data to calculate percent? 2) For each of the adult formulas of the drugs from example one, collect the data you would need to calculate the percentage of one dose that is made up of active ingredient. Show your work. Discuss it - II: 1) Did the percent active ingredient differ from one type of drug to the other? If so, why might this be? 2) Why do you think the medications contain inactive ingredients? In other words, what might be the purpose of including the other ingredients? Explore it - III: While some drugs are given as pills of dry powder, others are given as liquids. 1) Why might it be important or useful to make a medication into a liquid as opposed to a pill? 2) How might you turn a dry powdered medication into a liquid form of the medication? 3) Try making liquid Gatorade from powdered Gatorade using your procedure from number 2. Watch what happens to the powder and record your observations here. Discuss it - III: 1) Discuss with the class the results of your Gatorade experiment. Try to come up with a scientific term to describe the end result. 2) When we dissolve one substance into another substance we can use scientific terms to identify each one. The substance that is being dissolved is called a solute and the substance in which the solute is dissolved is called the solvent. In your Gatorade example from above, identify which substance was the solute and which substance was the solvent. Solute: ______________________________ Solvent: _____________________________ 3) If you added more Gatorade powder to your solution, what would that do to the concentration of that solution? (Hint: imagine how it would taste compared to the original solution- DO NOT ACTUALLY DRINK THE SOLUTION) 4) If you added more water to your original Gatorade solution, what would that do to the concentration of the solution. (Hint: again, imagine how it would taste compared to the original solution- DO NOT ACTUALLY DRINK THE SOLUTION) 5) Write an equation that shows how the words solute, solvent and solution are related. Explore it - IV: While we can describe the concentration of a solution as high or low, when applying this information to drug dosage, it is important to be more precise. One way that we can express the strength of a concentration is by calculating a mathematical expression called percent solution. We know that a percent is a way to represent ‘parts per 100;’ percent solution just applies this idea to solutions by describing the amount of solute in 100 mL of total solution as a percent. We use volume (in milliliters, abbreviated mL) as the unit of measure to describe how much total solution is present. However, because solutes are not necessarily liquid, we can use either weight (in grams, abbreviated (g) or volume (mL) to describe how much solute is present; therefore, we use two different methods to calculate percent solution depending on the state of matter of the solute. For example, some people use liquid laundry detergent while others use a powdered detergent. If someone switched between a liquid and powdered detergent, but still wanted to have the same strength of detergent, they could use percent solution calculations to ensure that they can keep it consistent. Weight/volume (W/V) percent solution o A mathematical expression to compare a solid solute (g) to 100 mL: of the total solution. o Formula: ____Mass of solute (g)_____ x 100 Volume of solution (mL) Volume/volume (V/V) percent solution o A mathematical expression to compare a liquid solute (mL) to 100 mL: of the total solution. o Formula: ____Volume of solute (mL)__ x 100 Volume of solution (mL) It is important to note that for both formulas, we are using the total volume of the solution, not just the volume of the solvent. Recall the equation from part two that you used to relate solute, solvent and solution. To further demonstrate the difference between the volume of the solvent vs. the volume of the whole solution, try the following and record your observations in the chart below. Obtain the following from your teacher: Salt Water 100 mL Beaker 100 mL Graduated Cylinder Scale Weigh boats Procedure: 1) Weigh out 5 grams of salt and pour the salt into the empty beaker. 2) Measure out 100 mL of water in the graduated cylinder. 3) Slowly pour water from the graduated cylinder into the beaker until the total solution in the beaker reaches 100 mL. How much water did you use? Record your data in the chart below. 4) Empty your supplies and repeat steps 1-3 two more times using 10g and then 20 grams of salt. Record your data in the chart below. Grams of solute mL of solvent Total volume of solution 5 10 20 Discuss it - IV: Use your data above to answer the following questions: 1) The total volume of the solution is the same for all three trials, but the amount of solvent used to produce that volume of solution is different. Explain why this occurred. 2) Which two columns would you use from the chart above to calculate the percent solution for the three trials? Explain how you would use the data. 3) If our solute was a liquid instead of a solid, how would your percent solution calculations differ? Apply it: You have learned a lot about how the vocabulary involved in understanding and calculating the strength of a solution (the concentration), now let us apply it to drug dosage calculations that you might use as a healthcare professional. 1) Your patient is extremely dehydrated, so you decide to administer 2000mL of a 12% saline solution to help rehydrate them. This is a common solution used in hospitals for this purpose that is made out of salt and water. How would you make this solution? Write using complete sentences and show your calculations. 2) In the solution described in number 1 above, which substance is the solvent and which is the solute? 3) How many grams of ampicillin (an antibiotic) would you need to prepare 500 mL of a 20% solution of antibiotic and water? Show your calculations and be sure to use the correct units. 4) Your patient needs 50 mL of antibiotic, but the antibiotic must be diluted to a 10% solution in water in order for the patient’s body to process it correctly. How much water would you need to add and what would the total volume of solution would you end up with. Show your calculations and be sure to use the correct units. 5) You have 10 g of a powdered antibiotic and you need to make a 30% solution; you need to add water to dilute it. What will be the volume of the final solution? Show your calculations and be sure to use the correct units. 6) A patient has been hooked up to an IV containing 1000mL of a 5% solution of antibiotic. The patient falls asleep and the IV falls out after only 600 mL have been administered. How much of the antibiotic (in mL) has the patient received? Show your calculations and be sure to use the correct units. Expand it: If all this is making sense, check out some resources to find out more about how doctors and other health professionals apply this type of information! Do some research either in a medical text book or using reputable internet sources to find more information to answer any of the following questions. How are IV solutions made commercially? How do IV pumps work. How can ‘percent solutions’ be applied to hemodialysis? What is a ‘gel cap’? How are dosing guidelines determined? What other ways can medication be administered besides in pill or liquid form? Are they produced in similar ways? Assess it: Problem: You are working as a researcher for a pharmaceutical company who is developing antibiotic treatments for hospitals. Your boss as put you in charge of a project to find the minimum dosage of an antibiotic that is effective in getting rid of a bacterial infection. You will be setting up an experiment to test out various percent solutions of two different antibiotics to determine how you can make the cheapest, yet most effective antibiotic solution so sell to hospitals. The two antibiotics that you will test are Kanamycin and Ampicillin. The Kanamycin is $100.00 for 5 grams of powder, while the Ampicillin is $200.00 for 5 grams of powder. In other studies that you have done, you have found that you do not need more that a 50% solution of either antibiotic to kill off susceptible bacteria. You will need to design an experiment with the following components: 1) Introduction/background, 2) hypothesis and predictions, 3) experimental design/procedure, 4) data, 5) conclusions. Follow this format and fill in your experiment below. 1) Introduction/background: Provide a brief background for your experiment. Use what you have learned in this lesson as well as information from reliable internet or book sources. 2) Hypothesis/predictions: 3) Experimental design/procedure: Explain how you will test your idea. Be sure that you include experimental (treatment) groups and at least one control group. Describe the actual procedure that you will use. Feel free to draw pictures to help describe the design, but be sure to label them. 4) Data: Explain what data you will collect. Be sure to include details such as what units you will use and how the data will allow you to answer your question. Explain how you will organize your data in order to analyze it. 5) Conclusions: Explain how you will analyze your data and provide examples of possible scenarios or outcomes along with an interpretation of what that outcome would mean for your question. In other words, describe how you will use the data to pick the antibiotic solution that fits the task that your boss at the pharmaceutical company gave you. After you have completed this task, brainstorm other ideas besides increasing the percent solution of an antibiotic to kill more bacteria; what other approach(es) could you use to create drug solutions to help patients fight off their infection?