SINTERING OF CERAMICS
advertisement
SINTERING OF CERAMICS BY: Mohammad Ali DEFINITION • Sintering commonly refers to processes involved in the heat treatment of powder compacts at elevated temperatures, where diffusional mass transport is appreciable. • Successful sintering usually results in a dense polycrystalline solid. However, sintering can proceed only locally (i.e. at contact point of grains), without any appreciable change in the average overall density of a powder compact. SINTERING A MODEL SKETCH BASIC THERMODYNAMICS OF SINTERING • Sintering is an irreversible process in which a free energy decrease is brought about by a decrease in surface area. • The driving force for sintering is a decrease in the surface free energy of powdered compacts, by replacing solid-vapour interfaces (of surface energy sv) with solid-solid (ss) interfaces, where ss < sv. BASIC THERMODYNAMICS OF SINTERING (contd.) • The change of system energy dE due to sintering is therefore composed of the increase due to the creation of new grain boundary areas, dAss > 0, and due to the annihilation of vapoursolid interfaces, dAsv < 0. • The necessary thermodynamic condition for the sintering to proceed is: dE = ss dAss + sv dAsv < 0 WHY CERAMICS HAVE TO BE SINTERED? Ceramic processing is based on the sintering of powder compacts rather than melting/solidification/cold working (characteristic for metals), because: • Ceramics melt at high temperatures. • As-solidified microstructures can not be modified through additional plastic deformation and recrystallisation due to brittleness of ceramics. WHY CERAMICS HAVE TO BE SINTERED? (contd.) • The resulting coarse grains would act as fracture initiation sites. • Low thermal conductivities of ceramics (<30-50 W/mK), in contrast to high thermal conductivity of metals (in the range 50-300 W/mK) cause large temperature gradients, and thus thermal stress and shock in melting-solidification of ceramics. WHAT HAPPENS DURING SINTERING • Increase of interparticle contact area with time • Rounding-off of sharp angles and points of contact • In most cases, the approach of particle centres and overall densification • Decrease in volume of interconnected pores • Continuing isolation of pores • Grain growth and decrease in volume of isolated pores SINTERING STAGES Three sintering stages are discussed here with major changes taking place against each stage. INITIAL STAGE OF SINTERING • Local point of contact formation or "fusion", without shrinkage of compact. This is accompanied by smoothing of the free surface of particles. • Neck formation at the contact point, with the resulting concave curvature at the neck, in contrast to the convex curvature on the particle surface. INITIAL STAGE (contd.) • If the relative green density after forming of the particle compact was 60%, the density after initial stage would be about 70% of the theoretical density (TD). INTERMEDIATE STAGE OF SINTERING • Neck growth, • Pores forming arrays of interconnected cylindrical channels • Particle centres approaching one another, with the resulting compact shrinkage. INTERMEDIATE STAGE (contd.) • The shrinkage in the intermediate stage can result in additional densification by as much as 25%, or to a total of about 95% of the TD. • During sintering, if the only material transport mechanism originates on the surface of particles, no compact shrinkage takes place. • In such case, a change of the shape and size of pores and particles is observed and commonly termed as grain growth or coarsening. FINAL STAGE OF SINTERING • Isolation of pores, i.e. relative density exceeding ~93% • Elimination of porosity • Grain growth FINAL STAGE (contd.) • The final sintering stage begins at about 93-95% of theoretical density, when porosity is already isolated. • Ideally, at the end of this stage all porosity is eliminated. • The complete elimination of porosity in the final stage of sintering can only happen if the grain boundaries remain attached to the pores. FINAL STAGE (contd.) • This favourable situation happens only if the pores follow the movement of the grain boundaries and are not trapped within grains. • This means that discontinuous grain growth (i.e. few grains growing at a very large rate at the expense of all other grains, trapping porosity on its path) must be stopped. • It is suppressed through grain growth limiting additives, such as secondary phase particles at grain boundaries, and/or appropriate time and temperature control of the sintering process. CHANGES OCCURING DURING SINTERING OF A WHITEWARE TRIAXIAL (silica-mullite-leucite) AT APPROX. TEMP. C Up to 100 Loss of moisture 100-200 Removal of adsorbed water 500 Oxidation of organic matter 575 Little overall volume damage 980 Start of shrinkage 1050-1100 Glass forms from feldspar, mullite grows, shrinkage continues 1200 More glass, isolation of pores 1250 Max. densification, pores at min.(60%glass,21%mullite,19%quartz) SINTERING CATEGORIES • Solid state sintering occurs when the powder compact is densified wholly in a solid state at the sintering temperature. • Whereas liquid phase sintering occurs when a liquid phase is present in the powder compact during sintering. • Transient liquid phase sintering is a combination of liquid phase sintering and solid state sintering. In this sintering technique a liquid phase forms in the compact at an early stage of sintering, but the liquid disappears as sintering proceeds and densification is completed in the solid state. ISOTHERMAL SINTERING • Pure ZrO2 and ZrO2+14wt%Al2O3 were subjected to pressure-less sintering in vacuum at 1100 C (0.4Tm) for different periods of time. • Nearly full densities have been achieved in all cases, with average grain sizes not exceeding 100nm. • Very small grain size(<30nm) found in case-2, due to homogeneous distribution of Al2O3 phase particles hindering grain coarsening by pinning of grain boundaries. TWO STAGE SINTERING • 2SS is able to refine microstructure and in turn it improves grain size dependent material properties. • 2SS simply modifies sintering route by firing sample at hi-temp follow by rapid cooling down and dwelling at lower temp. PROCESS CONDITION for Zirconia, heating at 1350 C, follow by 900 C is found to be able to achieve comparable hardness as iso-thermal sintering at 1500 C. SINTERING VARIABLES The major variables which determine sinterability and the sintered microstructure of a powder compact may be divided into two categories: Material variables, & Process variables. MATERIAL VARIABLES • The variables related to raw materials are said as material variables. • These include chemical composition of powder compact, powder size, powder shape, powder size distribution, degree of powder agglomeration, etc. • These variables influence the powder compressibility and sinterability (densification and grain growth). PROCESS VARIABLES • Process variables involved in sintering are mostly thermodynamic variables. • These variables include temperature, time, atmosphere, pressure, heating and cooling rate. SINTERING TEMP FOR SOME COMMON CERAMICS SINTERING ADDITIVES • Sintering additives are usually added to powders to enhance the sinterability and to control the microstructure. • Addition of Ni to W for improving sinterability. • Addition of MgO to Al2O3 for suppressing abnormal grain growth (as pinning agent) and improving densification. EFFECT OF MgO DOPING • The greater the amount of MgO added, the greater the linear shrinkage, and as a result the greater the density as well. • In the sintering process, both densification and grain growth are in a competition. i.e. the densification process is limited if mass transport occurs for grain growth, and vice versa. • Since the presence of MgO in Al2O3 reduces the grain growth, the mass transport is mainly for densification. Therefore, to some extent, denser ceramics can be expected for higher MgO dopings. The changes of linear shrinkage for various MgO doping concentrations with various sintering time (in log scale) BINDERS/LUBRICANTS • Sometimes organic binders such as polyvinyl alcohol are added to hold the green body together. • These burn out during the firing (at 200-350°C). • Sometimes organic lubricants are added during pressing to increase densification. • It is not uncommon to combine these, and add binders and lubricants to a powder, then press. • Improved densification reduces the sintering time needed. SINTERING KILNS • Tunnel kilns and periodic kilns are commonly used for ceramics sintering (firing). • In periodic kilns heating and cooling sintering stages are conducted according to a prescribed procedure. • In tunnel kilns the sintered parts are conveyed through different temperature zones. • Typical tunnel kiln has three zones: 1. Preheat zone for removing lubricant and other organic materials; 2. Sintering zone where the diffusion occurs; 3. Cooling zone where the sintered parts cool TUNNEL KILN ADVANTAGES OF SINTERING • • • • The parts produced have an excellent surface finish, and good dimensional accuracy. The porosity inherent in sintered components is useful for specialized application such as filters and bearings. Refractory materials which are impossible to shape using other methods can be fabricated by sintering with metals of lower melting points. A wide range of parts with special electrical and magnetic properties can be produced. CURRENT TRENDS Selective laser sintering (SLS) is a rapid process that allows to generate complex parts by solidifying successive layers of powder material on top of each other. • Solidification is obtained by fusing or sintering selected areas of the successive powder layers using thermal energy supplied through a laser beam. SPS (spark plasma sintering) THE END

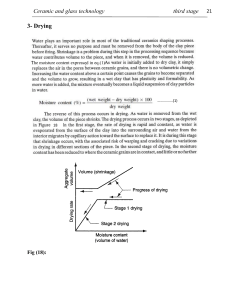





![[Sintering]](http://s2.studylib.net/store/data/005796423_1-9e9c09bb22b21ff2f3d3c85c8685e463-300x300.png)