Valence Electrons
advertisement
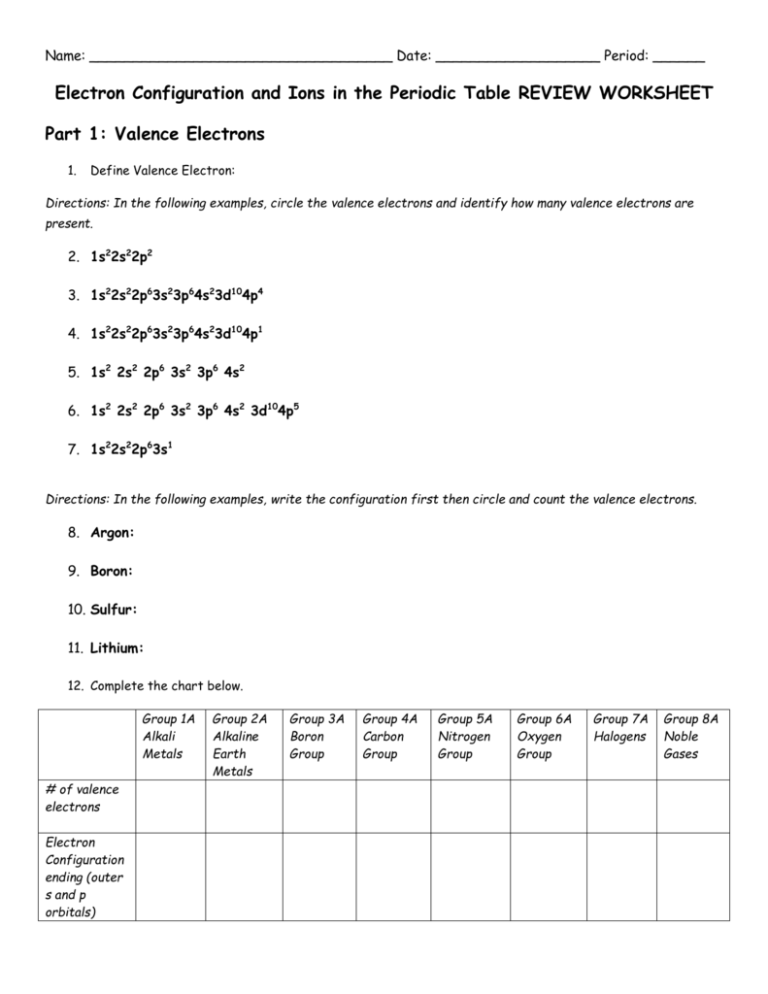
Name: ___________________________________ Date: ___________________ Period: ______ Electron Configuration and Ions in the Periodic Table REVIEW WORKSHEET Part 1: Valence Electrons 1. Define Valence Electron: Directions: In the following examples, circle the valence electrons and identify how many valence electrons are present. 2. 1s22s22p2 3. 1s22s22p63s23p64s23d104p4 4. 1s22s22p63s23p64s23d104p1 5. 1s2 2s2 2p6 3s2 3p6 4s2 6. 1s2 2s2 2p6 3s2 3p6 4s2 3d104p5 7. 1s22s22p63s1 Directions: In the following examples, write the configuration first then circle and count the valence electrons. 8. Argon: 9. Boron: 10. Sulfur: 11. Lithium: 12. Complete the chart below. Group 1A Alkali Metals # of valence electrons Electron Configuration ending (outer s and p orbitals) Group 2A Alkaline Earth Metals Group 3A Boron Group Group 4A Carbon Group Group 5A Nitrogen Group Group 6A Oxygen Group Group 7A Halogens Group 8A Noble Gases 13. The number of valence electrons is equal to the group number. Identify the number of valence electrons for the following atoms: a. Neon: _____ d. Calcium: _____ b. Chlorine: _____ e. Sulfur: _____ c. f. Potassium: _____ Aluminum: _____ Part 2: The Octet Rule 14. Define the octet rule: ______________________________________________________________ _______________________________________________________________________________ 15. An octet is equal to ____ (#) electrons. 16. Group 18 already have a stable electron configuration as they have ____ electrons in their highest occupied energy levels. 17. Atoms of __________________ (class of elements) tend to lose their valence electrons to leave a complete octet in the next-lowest energy level. Atoms of ________________________ (class of elements) tend to gain electrons or share electrons with another nonmetal to achieve a complete octet. 18. When the octet rule is satisfied, the outermost s and p ____________________ are filled. 19. The elements of the noble gas group satisfy the __________________ ______________ without forming compounds. This is why they are considered unreactive. 20. Atoms in a stable compound achieve the nearest noble gas configuration by ________________ electrons donating electrons, or by accepting electrons. 21. Atoms of nonmetals can achieve stable noble gas electron configuration by ______________ electrons. They end up having a _______________________ charge. 22. Atoms of metals can achieve stable noble gas electron configuration by _____________________ electrons. They end up having a _______________________ charge. 23. Identify whether the following atoms will want to lose electrons or if they will want to gain electrons. Pay attention to whether the atom is a METAL or a NONMETAL. Follow the first example. a. Na (11): Lose d. S (16): _____ g. b. F (9): _____ e. Mg (12): _____ h. Al (13): _____ c. f. N (7): ______ i. K (19): _____ Ca (20): _____ Cl (17): _____ Part 3: Ion Formation 24. What is an ion? _________________________________________________________________ 25. How is an ion produced? ___________________________________________________________ 26. A positively charged ion is a ___________________. A negatively charged ion is a _________________. 27. Which group of elements is most likely to become cations? _______________________. 28. Which group of elements is most likely to become anions? _______________________. 29. Fill in the blanks to determine what charge an ion will have. a. When a neutral atom loses 1 electron, it will become an ion with a ______ charge. b. When a neutral atom gains 2 electrons, it will become an ion with a ______ charge. c. When a neutral atom loses 2 electrons, it will become an ion with a ______ charge. d. When a neutral atom gains 1 electron, it will become an ion with a ______ charge. e. When a neutral atom gains 3 electrons, it will become an ion with a ______ charge. 30. Complete the chart below. Atom Name # of Valence Electrons Metal or Nonmetal Will it lose or gain electrons? # of e- to lose or gain Ion to be formed Fluorine (9) Potassium (19) Oxygen (8) Aluminum ( ) Magnesium (12) Nitrogen (7) 31. Write the electron configuration for each ion and determine what noble gas it represents Ion Mg 2+ Cl 1O 2Ba 2+ # of electrons in the ion Electron Configuration Noble Gas the Ion is representing





