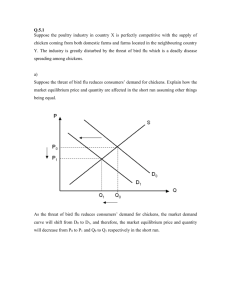
Infectious Stunting Syndromes – R. Reece
advertisement

Rod Reece Infectious stunting syndromes of chickens INFECTIOUS STUNTING SYNDROMES AND RELATED DISEASES IN POULTRY Dr Rod Reece, NSW Department of Industry & Investment, State Veterinary Diagnostic Laboratory, EMAI, Menangle NSW, Australia INTRODUCTION There are many causes of poor growth in broiler chickens, but in the mid 1970s a new syndrome, preferably referred to as ‘infectious stunting syndrome’ (ISS) appeared in Europe and USA, and thereafter spread rapidly throughout the world (Bracewell & Randall 1984). Mortality was not a feature, although cull rates were higher than normal. This syndrome was characterised by a 5-20% incidence (sometimes up to 50%) of stunting, with a few chickens being less than 50% the live-weight (LW) of more normal birds: severe runting (less than a third the LW of unaffected chickens), when present, usually affected only 1-5% of the flock. The affected chicks had voracious appetites and pendulous abdomens. Distension of the intestinal tract was noted on necropsy if they had recently eaten. Feathering was retarded. Affected flocks were usually detected at 4- to 14-days-old, and partial recovery of many affected chickens appeared to occur from 5-weeks-old. Oral inoculation of 1-day-old SPF chickens with intestinal homogenates prepared from affected chickens, induced a similar syndrome thus confirming this was a transmissible, ie infectious, disease. Following appearance of ISS on a farm or in a region, subsequent flock performance tended to be poorer than expected and there was intermittent clinical recurrence. Financial loss has been considerable due to poor feed conversion, excessive culling, reduced weight-for-age, greater than expected variation in weights at processing, and problems associated with processing and sale of small carcasses. Three distinct syndromes with separate target organs (namely, small intestine, pancreas, proventriculus) and thus different pathogenesis have been defined. They have been reproduced independently with bacteria-free inoculae, and it appears they have different putative aetiological agents (Reece 2001). ISS has been described as the “disease with too many names and faces” because there has been a tendency to ascribe names to the syndrome depending upon the clinical presentation, such as pale bird syndrome, helicopter chicks, runting and stunting syndrome etc. Numerous viruses have been isolated from and/or observed in the intestinal tract of affected chickens (including enterovirus, parvovirus, reovirus, rotavirus and togavirus), but an aetiological role has been difficult to ascribe for any of them. According to Koch’s postulates (actually Henle & Koch’s), a putative aetiological agent should be present in all affected cases and be absent from nonaffected cases; the presence of that agent ought to be sufficient to explain the clinical course and pathology of the disease; and isolated putative pathogens ought to be able to induce a similar disease in experimentally infected hosts, in this case chickens. As of 2010, no isolated putative aetiological agent has fulfilled these criteria and the only method of reproducing the disease remains oral inoculation of 1-day-old chickens with intestinal homogenates prepared from affected chickens, which in itself, is timeconsuming, costly and sometimes problematic. Unfortunately, the criteria for diagnosis of field cases have been poorly defined at times, and therefore clinical signs and/or pathology required for confirmation of experimental transmission have varied. The literature on this group of diseases has been difficult to follow! A consideration of Evan’s Association of Causation (see Appendix 1; Evans 1976) is enlightening: these were proposed to help elucidate pathogenesis and aetiology of complex and Date printed: 8/02/2016 page 1 of 24 Rod Reece Infectious stunting syndromes of chickens multifactorial diseases and were appropriate for ISS even though putative aetiological agents have not yet been isolated, let alone characterised. Funding for research into this group of diseases has tended to come from industry and the focus has been on projects that will last for only a few years but yield control programs that can be instituted by industry. This presentation is a compilation of the author’s experiences at Dept Agriculture, Veterinary Research Institute, Victoria funded by Chicken Meat Research Council; Institute of Animal Health, former Houghton Poultry Research Station, UK funded by Ministry of Agriculture, Fisheries & Food UK; and NSW DII, Elizabeth Macarthur Agricultural Institute, NSW funded by RIRDC-Chicken Meat. A similar syndrome was observed in other poultry industries especially turkeys and it was assumed these had a similar aetiology and pathogenesis: this may or may not be so. Some transmission studies utilising material from chickens were able to induce a stunting syndrome in turkeys, but on other occasions this has been unsuccessful. A somewhat similar stunting syndrome was reported in guinea fowl. A summary of the development, structure and function of the chicken gastrointestinal tract is attached as Appendix 2. ISS – THE INTESTINAL OR ENTERIC FORM EPIDEMIOLOGY There are many reports of apparent breeder flock association of stunting syndromes: this may be a reflection of vertical transmission and/or insufficient maternal protection and/or young age of dams with smaller chickens at hatch and/or some other factors. It is unknown if any such association would be due to on-the-egg (ie external, a from of horizontal infection) or in-the-egg (ie true vertical transmission). There is some evidence of variation in susceptibility of broiler chicken strains to ISS based on severity and nature of the inflammatory cell response EXPERIMENTAL TRANSMISSION STUDIES Semi-purified (free from enveloped viruses such as avian leukosis virus, reticuloendotheliosis virus, Marek’s disease virus and infectious bronchitis virus; as well as free from avian encephalomyelitis virus, infectious bursal disease virus, reovirus, adenovirus, chicken anaemia agent) bacteria-free intestinal homogenate (ISS-F) was able to induce typical intestinal form of ISS in chickens (with 100% of inoculated chickens having crypt lesions in mid-jejunum at 5dpi), but did not induce growth suppression nor intestinal lesions in turkeys, ducks, Japanese quail, pheasants nor guinea fowl. Intestinal lesions were similar in distribution and range of severity in several SPF chicken and commercial broiler strains. The exposure of inoculated chickens to periods of cold stress during brooding (daily at 15oC for one hour during the first week) did not accentuate intestinal lesions, but the effect of cold stress on growth rate was variable. Co-infection with either chicken anaemia agent (CAV) or avian nephritis virus (ANV) did not accentuate the intestinal lesions, but CAV induced musculature haemorrhages and more marked thymic and bursal atrophy than with ISS inoculae. Growth suppression of inoculated SPF Rhode Island red chickens was accentuated in males on high nutrient density feeds. Pancreatic degeneration was not a feature in chickens inoculated with this material, nor with more than twenty other intestinal homogenates as inoculae. Inoculation of chickens with intestinal isolates of reovirus, several avian nephritis virus strains, an astrovirus, (G-4260; AAF-7; HPRS-M), and ANV-related viruses isolated from intestinal tracts of chickens (Entero-PV2; Entero-3; EF84/700) induced mild intestinal histopathology but no detectable crypt damage. Inoculation of Date printed: 8/02/2016 page 2 of 24 Rod Reece Infectious stunting syndromes of chickens chickens with ANV-F isolated from ISS-F induced minor reduction in growth rate, mild non-suppurative infiltrates in the small intestinal propria, rare apoptosis of villus enterocytes and mild interstitial nephritis, but no crypt lesions. Chickens inoculated at 7 and 14 days-of-age developed significant intestinal lesions comparable to that observed in chickens inoculated at 1-day-old, but minor growth suppression was noted only in chickens inoculated at 7 days-of-age. Homogenates prepared from pooled intestinal tracts or various segments (duodenum, jejunum, ileum or caecae) were similarly effective in transmitting disease, as was pancreas (have to wonder about surface contamination); but homogenates prepared from proventriculus (back flow of intestinal luminal contents does occur), kidney and liver (the putative aetiological agent was observed in macrophages of the intestinal propria, and some such cells may have entered the venous portal circulation to the liver) only induced a low incidence of mild intestinal crypt lesions. Incidence and severity of lesions declined with decreasing 10xfold dilutions of intestinal homogenates after 1/100. Infectivity was retained after several passages through/in chickens. Others have demonstrated infectivity by oral dosing with litter from affected flocks. Chickens in-contact with other chickens inoculated with this material (ISS-F), grew as poorly as infected chickens and there were significant histological changes to intestines. Other workers have also demonstrated in-contact transmission. The progeny of hyper-immunised (?) breeders were fully susceptible to infection (any passive immunity may have been masked by over-infection; or maybe maternal antibody is non-protective?). Establishing an experimental model to exclude cross-infection, especially with ANV, was a problem that was, eventually, overcome. CLINICAL SIGNS The clinical signs in affected chickens were voracious appetite but poor growth; they had pendulous abdomens if they had recently fed, and there was sometimes faecal matting around the cloaca. Feathering was poor. Lameness due to osteodystrophy was noted in some affected flocks but was not a feature in experimental studies. In experimental studies, calculated daily growth rate was most marked in the first week (50-60% compared to controls), but was still significant in the third and fourth weeks (70-85%). PATHOLOGY Affected chickens were small and, although in poorer than expected bodily condition, they were not emaciated. On necropsy, if the bird had recently fed, the intestines were distended with poorly digested contents. The thymic lobes were small and often atrophic with a vestigial cortex, and the bursa of Fabricius was relatively small with small follicles. In field cases, various osteodystophies were observed in the physis of long bones, particularly the proximal tibiotarsus. In chickens receiving high carotenoid diets, the body was pale. Histologically, specific lesions were confined to the small intestine. These were more prominent and more pronounced in the mid jejunum than in distal duodenum or mid small intestine, were almost absent from terminal ileum, and not present in the caecae. Initially there was oedema of the villus coria and proria with infiltration by macrophages and lymphocytes; small loose clumps of heterophils in the coria and propria were occasionally noted. Individual degenerate enterocytes were observed on the sides of villi at 1dpi. The crypt cell mitotic rate increased, the crypts were elongated and somewhat serpentine, and there was basophilia of the crypt cell cytoplasm indicating hyperplasia and immaturity; in the early stage there was a short Date printed: 8/02/2016 page 3 of 24 Rod Reece Infectious stunting syndromes of chickens phase of decreased villus height (a transient villus atrophy) and some enterocytes were detected in mitosis on the lower third of villi. Individual degenerate crypt enterocytes were noted, along with degeneration of surrounding macrophages. By 45dpi many crypts were cystic and lined by cuboidal attenuated epithelium; some of these cystic crypts contained necrotic cell debris and/or mucus. Crypt abscesses containing plugs of degenerate heterophils were rare. The cystic crypts were mostly resolved during the next few days, and by 14dpi villus height appeared normal (see below). ISS was characterised by a massive increase in intraepithelial leukocytes, which was greater at 14dpi than at 5dpi. Monocytes and macrophages commonly infiltrated into the villus coria and propria, and were noted between enterocytes on villi and in crypts. Initially, large numbers of ChT8 (MHC-1 associated cytotoxic/suppressor) cells were associated with crypts containing degenerate enterocytes and/or were located near degenerate macrophages; whereas ChT4 (MHC2 associated helper/inducer) cells were located predominantly within the coria and propria. By 14dpi, germinal centres (B-cells) were forming in the coria and proria, and these were surrounded by a rim of ChT4 cells. The intraepithelial lymphocytes on villi and within crypts were ChT8 cells, and similar cells were present in the coria and propria, either loosely scattered or as clumps. The intra-epithelial ChT8+ve cells were further identified as predominantly αβ1-TCR2, whereas γδ-TCR1 cells were rare. Structurally, there were marked alterations to the intestinal villi and crypts. Such changes were best determined in overnight fasted chickens to remove artefacts associated with luminal ingesta; and morphometrical measurements were obtained by microdissection to overcome artefacts as a result of fixation and processing. Besides transient villus atrophy at 5dpi, but a slight elongation by 14dpi, there was crypt elongation, including convoluted crypts (which were difficult to measure). The number of crypts per villus decreased. Excess goblet cells were noted on the villi due to both an absolute increase (a result of hyperplasia) and a relative increase due to loss of enterocyte chief cells. Crypt cell proliferation rate was greatly increased, possibly in part being driven by cytokines produced and released by the inflammatory cell infiltrates. The resultant migration rate of enterocytes along the villi was greatly accelerated. There was fusion of villi resulting in a haphazard arrangement of projections into the intestinal lumen. Using small inert markers, the gastrointestinal transit time of ingesta was accelerated. There was a lower density of crypts per area, probably due to separation of crypts by oedema and inflammatory cell infiltrate. Even though the chickens were smaller, the intestinal length was increased, thus giving a greater length per unit weight or metabolic body weight. The mucosal brush border enzyme activities in the jejunum were significantly reduced in ISS-inoculated chickens compared to controls, especially disaccharidases and aminidases (thus reducing ability to break down and absorb carbohydrates and amino acids, leading to malabsorption and possibly initiating an osmotic-type diarrhoea), and glucosidase (reflecting a reduction of enterocyte protein synthesis) and maltate dehydrogenase (reflecting mitochondrial and cytoplasmic damage within enterocytes): these were more marked at 5dpi than at 14dpi. There was a slight increase in intestinal brush border alkaline phosphatase activity of inoculated chickens at 14dpi compared to controls but the significance of this was uncertain (elevated iAP may be protective against luminal acidity?). Taken together, these results indicated biochemical immaturity of villus enterocytes, probably as a consequence of accelerated migration rate along the villi (there was probably insufficient time in transit along the villi for effective biochemical maturation of the enterocyte brush Date printed: 8/02/2016 page 4 of 24 Rod Reece Infectious stunting syndromes of chickens border), thus impairing nutrient absorption, as well as damage to enterocytes per se (Dr Ed Hall, University of Liverpool UK). Circulating concentrations of growth hormone were greater in 5dpi inoculated chickens than in the controls, but similar in both groups at 14dpi, whereas insulin-like growth factor (produced by liver in response to growth hormone) was not significantly altered either in absolute or relative terms (Dr C.Goddard, Roslin Lab, UK). A sequential electron-microscopic examination was undertaken by Dr Judith Frazier (Houghton, UK) and summarised here. Small dense cytoplasmic inclusions containing crystalline arrays of picornavirus-like particles (approximately 20nm diameter) (PVLP) were noted within enterocytes on the sides of villi at 18 hours-pi, and in crypt enterocytes at 36 hours-pi. They occurred in the cytoplasm of macrophages between enterocytes and within villus coria and in propria around crypts from 2dpi. These PVLP inclusions were not observed after 5dpi. The small cystic crypts noted more prevalently in the early phase, were lined by attenuated squamous epithelial cells (which had few brush border microvilli) and intestinal myofibroblasts, joined together by occasional desmosomes, but these cysts lacked a distinct basement membrane. The larger cystic crypts were lined by cuboidal enterocytes on a basement membrane. Many of the dilated and cystic crypts contained cellular debris and occasional granulocytes. By 7dpi, there were fewer dilated and/or cystic crypts, and these declined in prevalence over the remaining period of the study (to 28dpi). Intraepithelial leukocytes between enterocytes in crypts and upon the sides of villi were noted at 3dpi and became extremely prominent from 11-28dpi: these were both lymphocytes and macrophages, From 7dpi, the fibroblasts and myofibroblasts in the propria around crypts, and especially within villus coria, were arranged in a haphazard manner, that is, not aligned parallel with the long axis of the villi. PATHOGENESIS The virus (PVLP) invaded and damaged villus and crypt enterocytes, rather than inducing confluent enterocyte necrosis with ulceration. This invoked a marked inflammatory response with lymphocytes and macrophages. Repair of crypts and attempted restoration of intestinal structure and function resulted in enterocytes on the villi that were biochemically immature and therefore inefficient at nutrient absorption. During the first week of life post-hatch, the gastro-intestinal tract of chickens adapts to ingested food, and infection of small intestines at this crucial stage damages structure and function and thereby has a significant impact on growth, feed conversion efficiency, and nutrient absorption and utilisation. AETIOLOGY Electron-micropscopy of affected intestines at 1-5 days of age from field and experimental studies, revealed small intracytoplasmic virus-like particles of 20nm diameter (PVLP), in degenerate enterocytes and associated macrophages. Morphologically, these resemble picronavirus but they have not been isolated or characterised further. Special staining confirmed they were RNA not DNA, which was as expected. We were unsuccessful in propagating this agent in chick embryos inoculated by a variety of routes and ages, nor in a broad range of primary chicken cell cultures, and various chicken, avian and mammalian cell lines. Oral inoculation of 1-day-old chickens with intestinal homogenates, and subsequent harvest of intestinal tract was the only method of propagation that was successful. TREATMENT AND CONTROL Treatment of affected flocks emphasised control of environmental management factors such as chilling and brooding temperature, treatment with multi-vitamins, and Date printed: 8/02/2016 page 5 of 24 Rod Reece Infectious stunting syndromes of chickens rigorous culling. As the disease was readily transmissible, control required tight biosecurity, attention to hygiene including stringent disinfection of affected sheds and their surrounds (hence the former recommendations for formalin fumigation etc), nonreuse of litter, all-in all-out operations etc, minimisation of brooding stress, and control of immunosuppressive virus diseases such as infectious bursal disease virus, Marek’s disease virus and chicken anaemia virus. Note that in other species, including humans, enteric disease at a young age has significant implications as far as overall health is concerned, with a greater incidence of subsequent intestinal and extraintestinal infectious diseases, and reduced productivity. ISS – THE PANCREATIC FORM Commonly referred to as runting and stunting syndrome, because chickens with pancreatic degeneration were very severely runted. See Appendix 3 for a review of structure and function of chicken pancreas. EPIDEMIOLOGY As with other forms of ISS, there was some perceived association with particular breeder flocks, but in most commercial flocks it was difficult to confirm that a particular affected chicken came from a particular breeder flock and that this was consistent across several affected flocks, but absent from others. The low and inconsistent prevalence of pancreatic degeneration made epidemiological understanding difficult. There was some association with an increased incidence in association with brooding stress, which was born out by experimental manipulation. EXPERIMENTAL STUDIES Oral inoculation of 1do chicks with one particular intestinal homogenate in VIC (Drs Don Barr, Peter Scott, Ian Smart et al; Veterinary Research Institute) and two in UK induced a low but variable incidence, of varying severity of pancreatic degeneration and severe stunting. This effect was retained by serial passage in chickens, but the incidence and severity varied. Cold stress during brooding caused a modest increase in the prevalence, but not severity, of pancreatic degeneration. The VIC studies, and prior studies in UK (Drs Malcolm Martland & Harley Farmer, Houghton) were complicated by similar pathology in controls: this was overcome in latter studies by strict separation of staff and facilities. Inoculated chickens were poorly grown as a group, with many small chickens, but only a low and variable percentage (5-45%) developed pancreatic degeneration. CLINICAL SIGNS Chickens with pancreatic degeneration were small, hence the term ‘runting’, and failed to grow. Feathering was retarded. Their appetite was voracious. The proportion of severely ‘runted’ chickens in an affected flock was low (<1-5%), although the incidence of pancreatic degeneration amongst selected chickens was high. Affected chickens were difficult to catch on farms as they were very active, and when chased frequently escaped underneath their larger flock mates. PATHOLOGY Grossly, chickens with pancreatic degeneration had significant bursal and thymic atrophy. The intestinal contents were voluminous, watery or mucoid, and poorly digested. The physes of the proximal tibiotarsi sometimes contained remnants of embryonic cartilage (this normally was removed by 3-5 days-old). In affected chickens more than 3 weeks-old, the pancreases (pancreata) were firm, and reduced to a thin pale strip in the duodenal loop. Convolution of the affected pancreases was noted but this was also observed in normal pancreases, and therefore was an unreliable indicator of pancreatic degeneration. Date printed: 8/02/2016 page 6 of 24 Rod Reece Infectious stunting syndromes of chickens Histologically, there was initially increased apoptosis of acinar epithelial cells. The earliest definitive lesion was at 7dpi with dilation of intralobular ducts and some luminal debris. Dilation of acinar lumina and cuboidal attenuation of acinar epithelial cells at 11dpi was accompanied by widespread individual necrotic acinar cells and infiltration into the adjacent interstitium by lymphocytes. Foci of acinar atrophy with dense lymphoid infiltrates were noted by 14dpi: the extent of degeneration varied from small areas at the distal tip to involving most of the organ. The pancreatic acini were replaced by proliferating ductule-like structures lacking zymogen granules in apical cytoplasm: most of these were proliferating ductule epithelial cells extending out along the intact basal lamina, but some were attenuated acinar epithelial cells. There was apparent sparing of acini close to Islets of Langerhans. Capsular fibrosis and stromal fibrosis of affected areas was prominent by 21dpi and well-formed lymphoid germinal centres were present in the stroma adjacent to affected areas. Dilation of ducts, lymphoid and macrophage infiltration into duct walls, and rarely, definitive duct obstruction were noted in affected pancreases. Small foci of ductules near the hillock of Vater, or within pancreatic lobes were present in affected and normal and control pancreases, and assumed to be incidental. Surface crush artefacts were created by cutting pancreases out of duodenal loops with scissors. Small foci of acute necrosis of the superficial gland were noted from time to time in affected and normal pancreases. These lesions were associated with small numbers of heterophils but were distinct from pancreatic lesions occurring in bacterial infected yolk sacs, avian encephalomyelitis, high pathogenicity avian influenza or adenoviral disease, and considered incidental to this pancreatic degeneration. In recent times (mid-2010), we have noted that some chickens more than two weeks old with significant pancreatic degeneration, have large rafts of filamentous bacteria in the crypts of the lower intestines – caecum, terminal ileum – similar bacteria are normally observed only in the lumina. This is not a feature in ‘normal’ chickens, but we see it from time to time on free range poultry and those with other enteric conditions – it is distinct from crypt spironucleosis. It may be a reflection of abnormal gut flora, either species profile, relative density, location etc (we prefer to describe this as ‘dysenterobiosis’) which may be a consequence of altered flow and motility of contents, different pH, changes in substrate due to maldigestion of ingesta, altered mucus characteristics etc. PATHOGENESIS Early reports suggested some similarity between this transmissible pancreatic degeneration and vitamin E / selenium deficiency, but that lacks inflammation. Protein deficiency also induces pancreatic acinar atrophy but does not lead to fibrosis and dense non-suppurative inflammation. Zinc toxicity also induces pancreatic necrosis and degeneration. None of these were considered related to this transmissible pancreatic degeneration. It was assumed that a virus affected the duct walls, leading to partial or complete obstruction of the duct lumen with back-flow of digestive juices up the ductules to the acini and thereafter initiating acinar atrophy. There was widespread acinar cell necrosis but it was sufficiently dispersed to allow retention of the basal lamina. Proliferating ductule cells could then extend along the still intact basal lamina until they reached the atrophying acini (this sequential process has been well studied following duct ligation in chickens and rodents). The lesion in this pancreatic degeneration was accompanied by significant inflammatory cell infiltration into the areas of degeneration, which was consistent with it being induced by a viral agent. In Date printed: 8/02/2016 page 7 of 24 Rod Reece Infectious stunting syndromes of chickens addition, during the early phase at 14 days-old or thereabouts, in experimental and field cases, many acinar epithelial cells were observed undergoing mitosis (this aspect may be related to the chickens being young). It was assumed that chickens with extensive pancreatic degeneration would have low pancreatic juice excretion and therefore be unable to optimally extract nutrients from ingested food. Chickens are able to survive with significant damage to the exocrine acini of the pancreas, but it appeared that regeneration did not appreciably replace lost acinar secretory cells in severe cases and severe runting resulted. AETIOLOGY The disease was transmissible using bacteria-free intestinal homogenates, and could be repeatedly passaged in chickens: therefore it must have a viral aetiology. The UK inoculae contained reovirus and avian nephritis virus, and in some cases also induced intestinal lesions typical of ISS (but chickens inoculated with semipurified inoculae – see above - did not develop pancreatic degeneration so the putative aetiological agents responsible for enteritis and that for pancreatic degeneration, are distinct and separate). Irregular cytoplasmic particles, 45-60nm in diameter were observed in the cytoplasm of epithelial cells lining pancreatic ducts of ‘normal’ and degenerate pancreases, as well as in jejunal enterocytes and chick embryo fibroblast cell cultures. The nature of this particle could not be determined (although it had some morphological similarity to a togavirus, it was never confirmed that it was even a virus) and its role as a putative aetiological agent of pancreatic degeneration was not established. A similar disease in salmon, pancreas disease, has been shown to be due to infection with an alphavirus, a togavirius. Pancreatic necrosis of salmonids is due to infection with a birnavirus. CONTROL As for the other forms of ISS. ISS – THE PROVENTRICULAR FORM This was commonly referred to as transmissible viral proventriculitis (TVP), or infectious proventriculitis stunting syndrome (IPSS). For a review of normal structure and function fo the proventriculus, see Appendix 2. EPIDEMIOLOGY This syndrome occurred as a widespread endemic in the Australian broiler industry from 1996-2001 and we were able to confirm it’s presence in NSW, NT, QLD, SA, VIC and WA, and in multiple companies: the severity of the disease varied. Since then (to mid 2010), we have intermittently observed cases, either in individual chickens from flocks without apparent growth related problems, or at high incidence amongst selected chickens from poor performing flocks. There has been some association with particular (young?) breeding flocks and it was more obvious in spring-summer. Experimental studies showed ready in-contact transmission. EXPERIMENTAL STUDIES Several distinct and separate batches of proventricular-intestinal homogenates prepared from affected chickens were capable of inducing significant growth retardation and a high incidence (95-100%) of typical proventricular lesions when inoculated orally into 1-day-old SPF or broiler chickens. Homogenised proventriculi harvested from affected experimental chickens also induced similar effect in subsequent experiments, thus confirming that the disease was transmissible. Centrifuged, chloroform treated homogenates were capable of transmission of disease. The inoculae contained the enveloped viruses Marek’s disease virus and infectious bronchitis virus (which were inactivated by chloroform treatment; this would also Date printed: 8/02/2016 page 8 of 24 Rod Reece Infectious stunting syndromes of chickens have inactivated any avian leucosis virus-J but the presence of that was never able to be confirmed), but all the inoculae contained chicken anaemia agent, reovirus and fowl adenovirus type 8, and avian nephritis virus was confirmed by histology and serology in some experiments. Some inoculae capable of transmitting typical proventriculitis contained infectious bursal disease virus: whereas others were free. Experimentally, inoculated chickens were 5-10% lighter than gender-matched controls at 28dpi unless there as significant intercurrent disease. In early studies, some inoculae also induced typical intestinal lesions of ISS, and one induced a low incidence of pancreatic degeneration. Some inoculated SPF chickens developed severe anaemia, and thymic and bursal atrophy, consistent with chick anaemia virus: others succumbed to inclusion body hepatitis from fowl adenovirus 8: and others had acute bursal necrosis typical of infectious bursal disease. Separation of a putative aetiological agent from this “soup” was not achieved. Several of these viruses were lymphotrophic and would be expected to be present in an intense inflammatory response where lymphocytes were abundant ie Marek’s disease virus, infectious bursal disease virus and/or there were large numbers of activated macrophages susceptible to chicken anaemia agent: but CAV and IBDV, and some of the other viruses found in these inoculate, can be difficult to isolate. Tissue culture or chick embryo inoculations tended to favour proliferation of reovirus and/or adenovirus. CLINICAL SIGNS Affected flocks had poor feed conversion, reduced growth rate and were excessively uneven in weights at slaughter age: a common scenario was of half the flock being several days behind in growth. Some affected flocks had an apparent increase in water consumption, and some had a significant problem with “wet litter”: however, “wet litter” was a problem in other flocks without apparent TVP. Clinical detection was not obvious until the third to fifth week. The feathering of affected birds was usually normal. Pendulous abdomens were not noted. Faecal matting around the cloaca was mild and affected birds were sometimes observed to void voluminous, wet, poorly digested faeces. PATHOLOGY The only consistent gross finding in affected chickens was confined to the proventriculus and consisted of thickening of the wall and white lobular mottling. The cut surface of the affected lobules often exuded clear mucoid fluid. Congestion of the tips of the proventricular folia was sometimes observed. Dilation of the isthmus into the gizzard was noted in some severely affected chickens. Dilation of the intestines with poorly digested feed of a semi-fluid consistency was noted if the birds had recently eaten. In some birds, the bursa of Fabricius and thymic lobes were smaller than anticipated. Histological lesions in the proventriculus were initially mild and somewhat subtle at 14dpi, but were pronounced by 28dpi. There was marked non-suppurative inflammation with infiltration of lymphocytes and plasma cells into the septa between and around glandular alveoli within the lobules, accompanied by numerous germinal centres. The alveoli were often cystic and lined by attenuated cuboidal or squamous epithelial cells: some crypts contained cellular debris. The tertiary ducts were lined by hypertrophied and hyperplastic epithelium, and extended out towards the edge of the lobules displacing or replacing the oxynticopeptic cells of the proventricular lobular alveoli. The lumina of the central ducts were often markedly dilated creating a cystic appearance which was noted grossly. The ductule epithelium was often multilayered, or arranged in piles, rather than pseudostratified. The connective tissue surrounding the central collecting duct was prominent with loose myxomatoid tissue containing Date printed: 8/02/2016 page 9 of 24 Rod Reece Infectious stunting syndromes of chickens numerous lymphocytes and macrophages, and germinal centres. The lamina propria under the surface, and the necks of the papillae, contained numerous dense foci or loose groups of mixed mononuclear cells. The mucosa of the folia appeared to have excess goblet cells, and mitoses in the epithelial cells at the base of the sulci were more plentiful than normal. There were many lymphocytes, often arranged as welldeveloped lymphoid germinal centres, and other cells, at the oesophageal junction of affected chickens. The koilin of the gizzard just beyond the isthmus was often poorly formed, with micro-ulcers, and foci of haemorrhage and heterophils (this also occurred in chickens without obvious TVP so we were uncertain if there was any real association). The proventriculi of chickens with overt Marek’s disease could be differentiated from those with TVP, and the gross and histological changes were distinct from proventricular dilation syndrome of chickens. Proventricular dilation of psittacines has been shown to be due to a bornavirus, but ganglioneuritis was absent from these cases of TVP. PATHOGENESIS The onset of growth retardation in TVP occurred later than in other forms of ISS. Severely stunted chickens was not a feature except with those with significant intercurrent disease. There was obliteration of proventricular alveoli and their oxynticopeptic cells thus leading, presumably, to a resultant decrease in hydrochloric acid and pepsinogen. In normal chickens, the oxynticopeptic cells released secretory granules via exocytosis, which then passed out into the alveolar lumen, and then this was moved along the ducts out into the proventricular lumen. The catarrhal (excess mucus production) reaction of the folia may have been a response to altered ingesta physico-chemical properties such as pH. We have no data on digestive juice productivity, let alone nutrient digestibility. We have no data on the impact of the inflammation and glandular damage on motility or discharge patterns of affected proventriculi. AETIOLOGY Electron-microscopic studies (Murkesh Srivastava, EMAI) revealed hexagonal viruslike particles 65-75nm in diameter in the nucleus, perinuclear region and in cytoplasmic vacuoles of proventricular alveolar epithelial cells in one submission of field material from 2 affected 6wo QLD broilers, and in the USA EM-grids supplied for confirmatory purposes. Sequential studies from several experiments, and many other field cases, did not reveal any virus-like particles besides adenovirus in the proventriculi of cases of inclusion body hepatitis associated with typical proventriculitis (these also had typical adenovirus inclusions in gizzard crypt epithelial cells). CONTROL Control has focused on clean-up between batches and adequate vaccination of breeder flocks against infectious immunosuppressive diseases. The move towards in-ovo vaccination of broilers against Marek’s disease may or may not benefit control of TVP. RECOMMENDED READING Bracewell, CD & Randall, CJ (1984). The infectious stunting syndrome. World’s Poult Sci J, 40:31-37. Rebel, JMJ, Balk FRM et al (2006). Malabsorption syndrome in broilers. World’s Poult Sci J 62:17-29. Date printed: 8/02/2016 page 10 of 24 Rod Reece Infectious stunting syndromes of chickens Reece, RL (2001). Infectious stunting syndrome and related diseases. In: Poultry Diseases, 5th ed. Eds Jordan, F, Pattison, M, Alexander, D & Faragher, T. Saunders, London: pp374-383. Dormitorio, TV, Giambrone, JJ & Hoerr, FJ (2007). Transmissible proventriculitis in broilers. Avian Path 36,2:87-91. Appendix 1. Evans,A.S. (1976). Causation and disease: the Henle-Koch postulates revisited. Yale Journal of Biology and Medicine. 49:175-195 Table 13. Criteria for causation: a unified concept. 1. Prevalence of the disease should be significantly higher in those exposed to the putative cause, than in case controls not so exposed a. 2. Exposure to the putative cause should be present more commonly in those with the disease, than in controls without the disease, when all (other) risk factors are held constant. 3. Incidence of the disease should be significantly higher in those exposed to the putative cause than in those not so exposed, as shown in prospective studies. 4. Temporally, the disease should follow exposure to the putative agent with the distribution of incubation periods on a bell shaped curve. 5. A spectrum of host responses should follow exposure to the putative agent, along a logical biological gradient from mild to severe. 6. A measurable host response following exposure to the putative cause should regularly appear in those lacking this before exposure (ie antibody, cancer cells), or should increase in magnitude if present before exposure: this pattern should not occur in cases not so exposed. 7. Experimental reproduction of the disease should occur in higher incidence in animals or man appropriately exposed to the putative cause, than in those not so exposed; this exposure may be deliberate in volunteers, experimentally induced in the laboratory, or demonstrated in a controlled regulation of natural exposure. 8. Elimination or modification of the putative cause or of the vector carrying it, should decrease the incidence of the disease (control of polluted water or smoke or removal of the specific agent). 9. Prevention or modification of the host’s response on exposure to the putative cause should decrease or eliminate the disease (immunisation, drug to lower cholesterol, specific lymphocyte transfer factor in cancer). 10. The whole thing should make biological and epidemiological sense. a Note that the putative cause may exist in the external environment or in a defect in host response. Appendix 2. THE DEVELOPMENT, STRUCTURE AND FUNCTION OF THE AVIAN TUBULAR GASTROINTESTINAL TRACT with particular reference to the DOMESTIC FOWL INTRODUCTION: The tubular gastrointestinal tract has a similar underlying structure in all vertebrates: namely, an inner mucosa, submucosa, muscularis and an outer serosa. The mucosa comprises epithelium, propria and muscularis mucosa. In avian species there are villi in the mucosa of caecum and colorectum. The mucosal epithelium rests on a basement membrane. The basement membrane anchors the epithelial cells onto the underlying connective tissue and with light microscopy, the Date printed: 8/02/2016 page 11 of 24 Rod Reece Infectious stunting syndromes of chickens basement membrane appears as a thin layer that can be demonstrated by PAS or silver stains. However, ultrastructurally the basement membrane consists of a reticular lamina synthesized by the underlying sheath or bed of interconnected myofibroblasts, and the basal lamina which contains extracellular matrix secreted by the epithelial cells themselves. The basal lamina consists of a clear lamina lucida abutting the epithelial cells and contains the glycoprotein laminin and other molecules which bind the plasma membrane, and the lamina densa containing type IV collagen fibrils which loop around underlying collagen bundles. The epithelial cells are attached to the basement membrane by basal hemidesmosomes. That portion of the proria within villi may be referred to as the ‘coria’, or corium if pleural; and if within large folds, plica or ridges the propria is a ‘trabeculae’, not a separating septa). The muscularis mucosa (often referred to as ‘muscularis interna’) is relatively thin and oriented longitudinally, except for in the caecum where there is an additional inner circular or oblique layer. In avian species, the submucosa is generally poorly developed. There are two layers of muscularis (often referred to as muscularis externa): an inner circular and an outer longitudinal. Between these may be observed small bundles of nerve fibres, the avian equivalent of Auerbach’s plexa (or myenteric plexi: the fine autonomic nerve fibres that innervate the intestinal smooth muscle). The external muscularis of the oesophagus is skeletal muscle. The serosa is the point of vessel and nerve entry and exit, and consists of mesothelium of low cuboidal to squamous mesothelium overlying a thin band of loose connective tissue. Thickened areas are associated with mesenteric attachments, usually on the dorsal aspect of the organ. MOUTH: Modern birds (neo-ornithes) lack teeth but food is picked up with keratinized beak or rhampotheca. The tongue moves food to the back of pharynx from where it is swallowed. There are complex branched salivary glands in the submucosa of the mouth (buccal cavity), pharynx and tongue: these secrete mucus but no digestive enzymes. The tongue is mobile but relatively rigid with a framework of skeletal bones from the anterior hyoid apparatus (these are cartilaginous in young chickens). Taste buds are present as solitary cells close to the surface of the oral or pharyngeal cavity; or as glandular buds associated with the ducts of the pharyngeal salivary glands, particularly at the base of the tongue. OESOPHAGUS: The oesophagus contains longitudinal lines of simple alveolar mucus glands which are more numerous and larger proximally: they discharge their contents to the oesophageal lumen via a short secretory duct. The necks of the glands frequently are associated with lymphoid aggregates. The surface epithelium is stratified squamous non-keratinised. The CROP is lined by stratified squamous nonkeratinised, non-glandular epithelium. This is a storage organ for food. After a short period of fasting, the first few boluses of food by-pass the crop and go straight to the proventriculus. Emptying of the crop is a reflex controlled by lack of fullness in the gizzard. Inflammation of the crop is ‘ingluvitis’. The crop in pigeons and doves is bilobed. At the oesophageal-proventricular junction there are dense lymphoid foci in mucosa, with numerous germinal centres and occasional deep crypts containing degenerate debris, or even microabscesses. The mucosal demarcation into the proventriculus is abrupt and without a definitive spincter. PROVENTRICULUS: The glandular lobules of the proventriculus traverse the deep mucosa and the submucosa with elements of the muscularis scattered at various levels Date printed: 8/02/2016 page 12 of 24 Rod Reece Infectious stunting syndromes of chickens throughout, including at times above the lobules. Each lobule is a compound tubular gland separated from its neighbour by thin trabeculae. The lobule consists of outer secretory alveolus (‘alveolus’ is a small cavity; whereas ‘acinus’ is the smallest lobule of a compound gland). The oxynticopeptic cells lining the alveoli secrete hydrochloric acid, pepsinogen and other proteolytic enzymes at the rate of approximately 8.8ml gastric acid per kg LW per hour in the domestic fowl (which is greater than equivalent mammalian species). They are a single layer of columnar to cuboidal epithelial cells with intercellular canaliculi which distend on fixation to create a serrated or crenated appearance: they lack lateral desmosomes to hold the cells together. In the replete state, these cells contain spherical secretory granules produced by the Golgi apparatus. The nucleus is spherical or ovoid, and displaced basally. After discharge, the cells are more elongate with the nucleus in the distal third to central portion of the cell. Replenishment occurs gradually within several hours of discharge. After feeding, secretory granules are discharged by exocytosis and may be observed in the alveolus lumina as small pink membrane-bound blobs. The alveoli empty into tertiary ducts lined by low cuboidal epithelium which connect to a central cisterna and thence via secondary ducts from each lobule which join to form a primary duct which discharges into the proventricular lumen via the excretory pore of the conical protrubrance or papilla. The prevalence of goblet cells in the ducts decreases towards the alveoli. There are often lymphoid foci adjacent to the duct at the neck of the papilla. The papilla is surrounded by circumferential folds or plica with intervening sulci. In routine fixed material, the mucosal goblet cells deep in the sulci are often compressed and may take on the (false) appearance of a simple alveolar mucus gland. In the proximal proventriculus, the surface goblet cells predominantly produce neutral glycoproteins. The cells lining the plica are columnar but decrease in size towards the base of the sulci; they have supranuclear mucin granules. A light but diffuse infiltrate of lymphocytes and some macrophages, with occasional heterophils, is common in the superficial propria; this area is also an occasional site of neonatal extra-medulllary myelopoiesis. A low proportion of whole grain can be added to the diet of broiler chickens to help reduce the incidence of proventricular dilation. The muscularis is well developed with prominent plexi between the layers. Proventricular contraction depends upon gizzard fill, but there is limited oscillation of contents. Little digestion actually occurs in the proventricular lumen. The isthmus connects the proventriculus to the gizzard or ventriculus. It is narrow and about 1 cm long. There is no spincter as such, although the proventricular glands terminate abruptly. The mucosa of this intermediate zone is an intermingling of the mucosa types, and the koilin here and in the proximal gizzard is often fenestrated. GIZZARD: The gizzard has a thick collagenous tendon aponeurosis spreading out from the centre of each side: this overlies the muscularis. The bulk of the muscularis is the circular layer; the outer longitudinal layer is vestigial and restricted to the lesser curvature, thus for much of the circumference, the myenteric plexus lies just under the serosa; there is an inner oblique muscularis layer. The gizzard is lined by ‘koilin’, an amorphous keratin-like protein matrix (sometimes referred to as ‘cuticle’). The mucosal surface is indented by deep pits which extend into the deeper glandular tubules (not crypts) as groups separated by fine septa in the propria. The chief cells lining the glandular pits are low columnar to cuboidal, with a round nucleus. The tubular lumina tend to be expanded deep in the pits, and pits with distended basal lumina are occasionally noted. Discharged granules can occasionally be observed Date printed: 8/02/2016 page 13 of 24 Rod Reece Infectious stunting syndromes of chickens between the microvilli deep in the pits. The chief cell secretion enters the glandular lumen as a fine filament which reacts with other secretions to form a compact mass which is thus anchored into the upper reaches of the glandular mucosa. Koilin matrix is deposited periodically, hence there are horizontal laminations corresponding to rest lines. The koilin hardens as it matures. On the surface there are numerous tooth-like projections coinciding with the vertical columns, and intervening indentations giving rise to groves observed on the surface. Thin streams of degenerate cells arise from the tips of the glands. Proteolysis occurs in the gizzard lumen where pH may be 2.5 (compared to intestinal pH 6-8). The gizzard contractions have been described as Phase A: thin muscularis contracts anterior to posterior to mix the contents; Phase B: 2-3 major peristaltic waves from anterior to posterior which move the superficial suspension into the duodenum; Phase C: thick muscularis contracts posterior to anterior to reflux luminal contents back into the proventriculus; Phase D: contraction is from anterior to posterior to move proventricular contents from proventriculus into the gizzard lumen. The demarcation of the gizzard from the duodenum (ventriculopyloric orifice) is on the anterior ventral right third of the gizzard wall and is a fold not a spincter; but the gizzard contents are a slurry and therefore supernatant and small particles in suspension, spill over into the duodenum. The narrow ring of tissue (0.5 cm) between the gizzard and the duodenum is referred to as the ‘pylorus’; the cells here are arranged as branched acinar glands that somewhat resemble gizzard glands but do not produce koilin, there are many goblet mucus cells, as well as neuroendocrine cells equivalent to mammalian G-cells. This initial duodenal region contains dense lymphoid tissue in the propria (pyloric tonsil). SMALL INTESTINE: ‘Ingesta’ is material taken into the body via the mouth. ‘Chyme’ is the semi-fluid, relatively homogenous, creamy material produced by acid acting on ingested food, and discharged from the gizzard into the duodenum. The small intestine comprises duodenum, jejunum and ileum. The duodenum has a descending proximal arm and an ascending distal arm, with the pancreas suspended between in the duodenal loop; termination of the duodenum is at the hillock of Vater (papilla duodenalis) where the separate pancreatic and bile ducts penetrate through the intestinal wall and open into the lumen. The jejunum and ileum are of approximately equal length and demarcated by Meckel’s diverticulum (vitelline diverticula) which is the remains of the yolk sac (there is a dense lymphoid accumulation with numerous germinal centres at this site in chickens more than a few weeks old). Post-hatch there is a change from dependence upon yolk for nutrients, to digestion, absorption and assimilation of nutrients from ingesta, and this is associated with a rapid increase in digestive capacity after exposure to ingested food. Between late embryonic stage and adulthood, the internal surface of the small intestine increases by 50x. The intestine is relatively small until about 18 days of incubation, at which stage there commences a series of waves of villus formation arising from parallel crypt plates. This continues for a short period post-hatch. The number of villi is relatively set at hatch, there being about 24,000 in the duodenum of a chicken. At hatch, the villi are all finger-like projections, but by 10 days of age the villi in the duodenum have become large plate-like structures; whereas distally in the jejunum and ileum, the villi are progressively narrower and shorter, but are not fully formed until about 4 weeks of age. In domestic fowl, the villi are arranged obliquely to each other in a zig-zag pattern set at 40-60o to the longitudinal axis of the intestine. Villi increase in height and width post-hatch, but the greatest relative increase in height and volume occurs in the jejunum, not the duodenum even though the villi in the latter are Date printed: 8/02/2016 page 14 of 24 Rod Reece Infectious stunting syndromes of chickens longer. In the two days post-hatch, the length of the duodenal villi double in a normal chicken, but the microvilli of the brush border take 7 days to double in length. Intestinal mass increases six-fold in the first week post-hatch in a normal chicken and attains a plateau relative to live-weight at about 10 days of age. Increase in villus volume reaches maximal rate by 4 days in the duodenum, and 10 days in jejunum and ileum. At hatch, the villus enterocytes are round with a central nucleus, but after a few days they become columnar with a basally situated nucleus and a well developed brush border. Digestive capacity reaches maturity in the duodenum at about 7 days, but not in the jejunum until 14 days; which is several days after the peak increase in villus volume of the respective segment. Migration time for enterocytes to move from the crypt to tip of villus is similar in each part of the small intestine (3-4 days), but as the villi in different segments are significantly different in lengths, the actual migration rates markedly differ. Broiler chickens have larger villi and therefore more enterocytes in total, than layer type chickens of the same age, and the crypts are deeper, but relative increase in size with age are similar in different breeds or strains. Some minor variations in ingesta passage time and digestion rates in the early period post-hatch has been noted between strains, but it appears that absolute uptake of nutrients is largely determined by feed intake. The size and volume of villi and crypts is less in germ-free chickens than in conventional chickens, and the migration rate of enterocytes is much slower, probably a direct consequence of absence of bacterial activity in the intestinal lumina causing loss of enterocytes. Prior to hatch, crypts are not readily discernible, but immediately post-hatch, the crypts invaginate deep into the propria, accompanied by marked proliferation and differentiation of crypt enterocytes. Mitoses of enterocytes on villus sides are present for the first few days post-hatch and may be observed up to 10 days post-hatch, but thereafter should cease unless there is significant intestinal disease. Crypt numbers increase x7 post-hatch by branching and hyperplasia, especially in the duodenum, but the rate declines to negligible by 5 days. Crypt cell proliferation rate is a product of cell cycle time, the proportion of crypt cells devoted to proliferation and the crypt cell population size: 50% of crypt cells in the duodenum undergo mitoses in a 24 hour period. In the mid jejunum, the trench supplying each wide side of the villus contains 6-8 crypts: there are more in the duodenum and less in the ileum. Stem cells in the base of the crypts of Lieberkuhn produce three lines of enterocytes: chief cells, mucous goblet cells and enteroendocrine cells. (In mammals, the Paneth cell is an epithelial cell located deep in the crypts: these have large eosinophilic cytoplasmic granules and secrete ‘defensins’ and other substances, but such cells have not been conclusively demonstrated in the domestic fowl). Mitoses in the crypts are prominent, but mitotic figures on the sides of the lower villi are only found for the first few days post-hatch in normal chickens, and should cease by 10 days of age. The enterocytes migrate out of the crypt, move along the villus in a conveyor-belt-like fashion, and then exfoliate (slough-off or are shed) into the lumen (these cells often referred to as ‘effete’). The enterocytes are lightly attached to the tips of the villi and are easily displaced by rough handling or flushing; oedema accentuates this tendency for separation off the basement membrane, and post mortem change is more rapid and more marked in inflamed gastrointestinal tract than in normal tissue. Chief cells commence in the crypt as cuboidal ‘poorly differentiated enterocytes’, and these secrete water and electrolytes into the crypt lumina. They have short and sparse microvilli. The maturing villus chief cells are columnar, with an apical dense brush border composed of long microvilli. The nucleus is oval and Date printed: 8/02/2016 page 15 of 24 Rod Reece Infectious stunting syndromes of chickens located in the lower third of the cell. A pale area above the nucleus is due to the Golgi apparatus and its associated granules, but prominence, or lack of it, is cyclical during digestion. The cytoplasm is rich in mitochondria and also contains many small vesicles. It is useful to consider that the epithelial cells have three separate domains: apical, lateral and basal. The apical cytoplasm lying immediately under the brush border is largely devoid of cytoplasmic granules and contains a fine filamentous meshwork through which pass the rootlets of the microvilli; this is the ‘apical web’. The lateral region of chief cells are joined to their neighbours near the apexes by means of a tight junction, the ‘zonular occludens’, and underlying that a ‘zonular adherens’; there are many desmosomes along the lateral wall to bind the cells together. This forms a seal between the intestinal lumen and the intercellular space but it still permits movement of water and small molecules. The lateral walls of adjacent cells also interdigitate with each other. The basal cell membrane forms frequent blunt penetrations through the basement membrane into the underlying corium or propria. The mucous goblet cells develop from the crypt oligomucus cells and have a basal nucleus with the bulk of the cytoplasm being distended with secretory material in a membrane bound globule. Goblet cells are more numerous in the crypts than on the villi, and they are relatively more numerous in the distal intestine than proximally, and individual goblet cells of lower intestine are larger than those more proximal. The mucus is secreted from the cell by exocytosis involving the apical membrane. Following discharge of their contents, goblet cells may appear compressed by their neighbours. Just prior to hathc, the intestinal goblet cells produce predominantly acid mucin, whereas by 7 days post hatch, there are similar amount of neutral acetylmucins and acid mucopolysaccharides (sialomucins and sulphonomucins) produced in the intestinal goblet cells: note that individual goblet cells in the small intestine may produce either sialomucins, or sialomucins plus suphomucins. The mucous layer of the small intestine contains mucus, water, serum, cell debris and sometimes bacteria, and protects the underlying epithelium, lubricates luminal content and facilitates transport of nutrients between the lumen and the epithelial cells. Mucous assists the digestion, transport and uptake of specific nutrients, and the exclusion of microorganism and toxins. Enteroendocrine cells (‘enterochromaffin cells’) are infrequent in the crypts, and rare on the sides of villi (‘entero’ to denote their origin; ‘chromaffin’ for their having an affinity for chromium salts, also described as argentaffins and argyophils as the cytoplasmic granules can be highlighted with silver stains). They are pyramidal in shape with a wide base on the basal lamina. Small eosinophilic granules occur in the basal cytoplasm and adjacent to the nucleus. These cells secrete serotonin, and other substances, which stimulates glandular secretions and intestinal peristaltic activity. Endocrine cells in the chicken intestinal tract produce a variety of substances which modulate secretory, motor, absorptive, and other processes. The relationship of these with gastrointestinal function is complex. Neurendocrine cells in the pylorus (see above) liberate gastrin which increases acid secretion by the proventriculus; pancreatic polypeptides also increase proventricular acid secretion. Gastrin-releasing peptide is released by post-ganglionic fibres of the vagus nerve that innervate the Gcells of the pylorus. Bombesin released by proventricular mucosal endocrine cells stimulate the release of gastrin, enhances gut motility and increases pancreatic secretion. Neurendocrine cells with long cytoplasmic processes coursing parallel to the long axis of the proventricular glands but beneath the basal lamina, are concentrated in the middle of the lobules and secrete somatostatin (a neurotransmitter Date printed: 8/02/2016 page 16 of 24 Rod Reece Infectious stunting syndromes of chickens that inhibits the release of peptide hormones, in this case focused on decreasing the production other hormones from the intestinal tract and pancreas. The propria contains blood vessels, lymphatics (but not a central villus lacteal as in mammals), nerves, a fine network of reticular fibres, and a well developed core of smooth muscle fibres tending to be aligned parallel to the long axis of the villi. Nutrient supply post-hatch is crucial for small intestine development. Delayed access to feed post-hatch arrests the development of the intestinal mucosa and results in an increased proportion of proliferating enterocytes but reduced villus height and volume, reduced crypts per villus, and reduced enterocyte migration rate, and an increase in acid mucin production. Additionally, post-hatch feed deprivation lowers activity of intestinal digestive enzymes, especially disaccharidases, and this may persist for several weeks despite subsequent access to adequate feed: this is most marked in the jejunum. Post-hatch utilization of dietary fats is limited by initial low pancreatic lipase secretion. The pH of the intestinal tact ranges from 6.4 in duodenum to 8 in distal ileum. Significant changes occur in the intestines of chickens with regards to digestion and absorption of carbohydrates and other nutrients during the first weeks after hatch, coinciding with a shift from utilization of yolk reserves, to reliance upon ingested food. The nutrient transport systems exhibit independent developmental patterns in the first few weeks post-hatch, each potentially altered by genetic, hormonal, dietary and/or other factors. Digestion of macromolecules occurs in the lumen of the gastrointestinal tract, with the final stage of digestion occurring by membrane anchored enzymes in the enterocyte brush border, usually involving active cojoint Na+ absorption. Once a nutrient is absorbed by an enterocyte, the excess Na+ must be removed by baso-lateral membrane Na/K dependent adenosine triphosphatase ‘sodium pump’. The cells export 3x Na+ and import 2x K+, thus creating low intracellular Na+ concentration and an electrochemical gradient across the cell. This also powers the carrier systems to export monosaccharides. It has been estimated that each mature villus enterocyte in the rat jejunum contains 150,000 such ‘sodium pumps’, and a similar number are probably present in a mature chicken enterocyte. During the digestive process massive amounts of water are actively secreted into the intestinal lumen, and then resorbed; the presence of chyme in the lumen also creates an osmotic pressure which increases dramatically with macromolecular breakdown. In the crypts, enterocytes secrete Cl- via AMP-dependent chloride channel into the lumen: interference with this process results in massive water loss and fluid diarrhoea. Unlike neonatal mammals, the intestine of the recently hatched chicken is not able to absorb intact proteins or globulins. Proventricular pepsin and pancreatic trypsin present in the lumen of the intestine, convert dietary proteins into small peptides. Peptidases are anchored in the enterocyte brush border: these hydrolyse oligopeptides into free amino acids. The majority of amino acids are absorbed by means of Na+ dependent amino acid transporter proteins present in the apical plasma membrane. There is first the uptake of Na+, then the amino acid from the surface; the complex undergoes a conformational change, and then the amino acid is dumped into the cell cytoplasm. There are other amino acid transporter proteins that are Na+ independent, or H+ dependent. The amino acid diffuses through the cytoplasm, and the basal membrane then exports the amino acid via different Na+-independent transporters, into the extracelluar space for whence it enters the circulation. Gammaglutamyl-transferase is one such brush border enzyme required for amino acid transport across the apical membrane of enterocytes; activity is stimulated by dietary protein, activity peaks at about 5 days post-hatch, and the jejunum has a higher Date printed: 8/02/2016 page 17 of 24 Rod Reece Infectious stunting syndromes of chickens concentration than the duodenum or ileum. A few simple dipeptides are able to be absorbed without being first separated into their constituent amino acids, but these are broken down by cytoplasmic peptidases. Dietary carbohydrates are degraded by pancreatic amylase in the intestinal lumen into disaccharides such as sucrose, maltose etc. These diffuse until they come into contact with the brush border and there the disaccharides are broken down by brush border anchored disaccharidases. The final product, glucose, galactose or fructose are moved from the intestinal lumen across the apical membrane via Na+dependent hexose transporters. Sodium dependent glucose/hexose transport in the brush border peaks at 2 days post hatch. Luminal lipids such as triglycerides are broken down by pancreatic lipase and solubilised into micelles (bile emulsifies the products of lipolysis to form micelles, a complex of bile salts, phospholipids cholesterol etc). These diffuse through the mucus film and thence through the enterocyte apical membrane as long-chain fatty acids or monoglycerides. They are then re-esterified into triglyceride in the smooth endoplasmic reticulum, complexed to apoprotein produced in the rough endoplasmic reticulum, and excreted via the Golgi apparatus as chylomicrons through the basolateral cell membrane as exocytotic vesicles into the extracellular space. These are then transported via the hepato-portal venous system to the liver, not via the lymphatics. Absorption of fat soluble vitamins A, D3 and E is also as micelles. Calcium transport from intestinal lumen is concentrated in the duodenum and dependent upon functional 1,25-dihydroxy-vitamin D3. Ferritin is a cytosol ironbinding protein present in many cells, including enterocytes of the proximal intestines, but it plays little role in iron regulation. Iron uptake from the intestines is regulated, perhaps not as tightly as in mammals. The mechanisms involved in this uptake are poorly understood, although receptor-mediated endocytosis is involved. Other micronutrients have their own specific mechanism of absorption. The intestinal nutrient transport systems are associated with membrane surface area, cellular metabolism and ion electrochemical gradients which drive uphill the transport of certain nutrients, density and turnover of membrane-bound carrier proteins, and carrier protein affinity for substrate. Optimal use of dietary nutrients by the chicken depends upon the efficiency of digestion and absorption, and these are closely related to rate of passage of ingesta through the gastrointestinal tract. Movement of material into the duodenum is dependent upon the physical characteristics of the ingesta in the gizzard, hence the presence of whole grain reduces dilation of the isthmus, whereas transit through the proventriculus and duodenum is independent of physical characteristics. Ingested particles approximately 1mm in diameter first appear in the faeces after about 2.5 hours, and 90% are voided by 12 hours. Intestinal motility in the domestic fowl is by caudal moving peristalsis to mix and move the ingesta; retroperistalsis occurs in the colorectum, and to some extent in the small intestine itself. Immediately post-hatch, the young chick is dependent upon the lipids and proteins of the egg yolk to supply essential nutrients, with the yolk normally depleted by 4-6 days. Much of these nutrients are absorbed by endoctyosis direct into the blood supply, but there is also a component of discharge via the patent yolk stalk into the small intestine, probably stimulated by intestinal peristalsis as ingesta flows through the intestines. By 3 days post-hatch, the opening is occluded by compression from lymphocytes. Interestingly, chickens and turkey poults deprived of feed and water post-hatch for a few days, have reduced uptake of yolk reserves (not increased, even though they are nutrient deprived). Date printed: 8/02/2016 page 18 of 24 Rod Reece Infectious stunting syndromes of chickens It is important to remember that in intestinal disease, the rate limiting step for the absorption of nutrients is usually not enzymatic digestion, but transport across the brush border of the enterocytes. Damage to the intestinal tract during the formative first few days post hatch has significant and often lasting impact on the growth rate and subsequent nutrient absorption capability of the chicken. Preparation and handling of the intestines for histological examination requires care. Age and breed should be given to allow interpretation. The segment of small intestine to be examined should be identified. Do not attempt to expel or extrude the luminal contents. Overnight fasting reduces gut fill compression of the long villi. Cut 2cm tubes of the intestine for fixation. Wider segments of intestine can be opened carefully, laid on cardboard and fixed. Fixing the entire gastrointestinal tract in situ may lead to poor penetration of fixative, and certainly the coelomic cavity divisions need to be broken down, and larger portions such as gizzard opened, if not removed and fixed separately. Bouin’s is a useful fixative, but tissues must be transferred to 70% alcohol after a few hours. Autolysis of the small intestines is rapid, especially in the duodenum ie major changes occur within 5 minutes. Morphometrics on routine fixed and processed sections is unreliable due to artefacts. CAECUM: The caecae are a pair of blind-ended sacs which extend cranially from their ileo-caecal junction. Initially, the lumen is narrower than that of the terminal ileum, but in the mid body it is about twice the diameter of the adjacent ileum. This restricted orifice facilitates entry into the caeca of fluid and small suspended materials. The muscularis is relatively thin. In the proximal caecum, the villi are well developed but they are much shorter in the mid body and almost flat in the distal caecum. The crypts are relatively shallow. The caecum has longitudinal mucosal folds. The proportion of goblet cells in the epithelium decreases distally. In the medial wall of the caecal, within the mucosa are found the caecal tonsils consisting of dense lymphoid accumulations and germinal centres: these occur in the propria and also the villus coria, and may be associated with deep crypts some of which may contain cellular debris and even groups of heterophils (so-called crypt abscesses). There is also a dense cluster of lymphoid tissue at the distal extremity of the caecum. The caecae are filled by peristalsis in the intestine driving ingesta towards the caecum, and retorperistalsis in the colorectum driving material from there back up towards the caecal opening. The restricted orifice size and the presence of caecal villi probably filter out larger particles. The caecal movements mix the contents and move them towards the distal caecum; this continues until the caecum is full. Several times per day, waves of retro-peristalsis empty the contents into the colorectum. Caecal droppings are viscous, semi-liquid and usually brown, and are distinct from the more cylindrical, and more frequent intestinal droppings. The luminal contents of the caecum are thus relatively stationary for considerable periods and there is massive proliferation of the luminal flora which is able to metabolise some of the ingesta that ends up here. The mucosa absorbs considerable amounts of sodium and water, some amino acids derived from microbial proteins, and short-chain fatty acids from microbial degradation of complex polysaccharides such as cellulose. COLORECTUM: The colorectum in domestic fowl is short and straight with a thick muscular wall. The inner surface is subject to much distension and may become folded; the villi are short, but may appear flattened depending upon luminal fill at the time of fixation. Goblet cells are abundant. The crypts are short. There is a dense layer of lymphocytes and macrophages under the mucosal epithelium in normal birds. Date printed: 8/02/2016 page 19 of 24 Rod Reece Infectious stunting syndromes of chickens CLOACA: The cloaca is the common opening of the digestive, reproductive and urinary tracts. The cloaca is divided into three distinct cavities separated by circumferential contractile rings: the cranial coproderm is an ampulla continuous with the lumen of the colorectum and acts as a receptacle for faeces awaiting voiding; this is separated from the uroderm by a thick flap (plica coprourodealis). The ureters from the kidneys, and the slit from the vagina or vas deferens, open through the dorsolateral wall of the uroderm, which in turn is separated from the larger proctoderm by a small ridge (plica uroproctodealis). The coproderm is lined by low columnar epithelium similar to that of the colorectum; the uroderm is lined by pseudostratified cuboidal epithelium; and the proctoderm is lined by stratified squamous epithelium. The opening to the exterior, the vent, has a dense muscular spincter and the lips are inverted into the proctoderm. Mounted on the crest of the ventral vent lip is the male phallus. The bursa of Fabricius is situated in the dorsal proctoderm wall, and dense lymphid foci occur in the proctoderm mucosa. In the domestic fowl, faeces from the colorectum are voided several times per hour. A fluid suspension of urates from the kidneys are regularly deposited into the uroderm, but reverse peristalsis, which is more-or-less constant in the colorectum, drives this fluid, and other contents, back up into the intestinal tract, and some of it arrives in the proventriculus. Of the 500ml of urinary fluid expelled into the uroderm daily by a 2kg chicken, 100ml is absorbed via the caecum and 300ml from the colorectum. INTESTINAL IMMUNE FUNCTION At hatch, there is little structured lymphoid tissue in the gastrointestinal tract besides the caecal tonsils and Meckel’s diverticulum. However, there is a basal population of T-cells and B-cells, and that is markedly expanded at about day 4 post-hatch due to a wave of lymphocytes from thymus and bursa. In older chickens, there are the well developed caecal tonsils and the lymphoid foci associated with the Meckel’s diverticulum. Numerous lymphoid germinal centres (gut associated lymphoid tissue, GALT) are scattered throughout the propria and coria of the intestine. They are concentrated as Peyer’s-patch-like accumulations where there often is also expansion into the underlying submucosa: these tend to occur on the mesenteric side of the ascending arm of the duodenum, on the mesenteric (dorsal) aspect of the distal ileum opposite the distal third of the caecum, and as several small concentrations in the antimesenteric side of the jejunum of growing chickens. The latter accumulations tend to be randomly distributed and few persist into adulthood. They contain primary lymphoid follicles, secondary lymphoid B-cell follicles or germinal centres, and a dense interfollicular T-cell population. The overlying epithelium is flatter and contains antigen-presenting M-cells with apical surface microfolds (hence ‘M’ in their name) rather than microvilli: these are able to take up organisms, particles, antigens etc from the intestinal lumen by endocytosis and phagocytosis, and transfer them on to underlying T-cells and dendritic cells. (Casteleyn et al 2010. Locations of gutassociated lymphoid tissue in the 3-month-old chicken: a review. Avian Pathology 39:143-150) There are no Paneth cells in the avian intestine, but defensins and other immunologically active substances are produced by macrophages and heterophils. Plasma cells in the intestines produce IgG (the most predominant antibody in the cytoplasm of enteric lymphocytes), IgM and IgA; IgA is the predominant antibody secreted in the bile. IgA is produced by plasma cells, taken up by a receptor in the Date printed: 8/02/2016 page 20 of 24 Rod Reece Infectious stunting syndromes of chickens enterocyte basal membrane and moved into the cell cytoplasm, transported to the apex, and then actively secreted into the intestinal lumen. Intraepithelial lymphocytes are relatively common, especially towards the apical region of the villi, and most of these are ChT8 (cytotoxic suppressor T-cells involved in MHC Class1 restricted interactions), and most of those are γδ-TCR1+ve; few are αβ-TCR2+ve ChT cells. Small numbers of natural killer cells, macrophages and heterophils may also occur as intraepithelial leukocytes. Globular leukocytes (shared lineage with mast cells) are relatively rare in normal chickens, but may be found in propria around crypts, in coria, and as intraepithelial leukocytes. The proria and coria also contain B-lymphocytes arranged as primary or secondary (germinal centre) follicles, plus ChT4 cells (helper-inducer T-cells involved in MHC Class2 restricted interactions), often accumulating peripheral to developing germinal centres. Toll-like receptors (‘toll’ was the exclamation made by the German workers who discovered this gene is Drosophila, being translated as ‘wow’ or ‘great’; something equivalent to Archemedes’ ‘eureka’) are part of the innate immune system and are a class of surface protein molecules of eukaryotic cells that detect and respond to conserved microbial molecules such as gram-negative cell wall lipopolysaccharide, flagellin or double stranded viral RNA. They alert the immune system by releasing cytokines, inducing apoptosis, encouraging phagocytosis or producing interferon (depending upon their cell type). They have been identified in the chicken intestine. There is some evidence that a tolerance-like phenomena can be induced by oral antigens in chicks up to 3 days post-hatch. GASTROINTESTINAL FLORA OF THE DOMESTIC FOWL MUTUALISM the flora benefits both the host and the microbes. COMMENSALISM either the host or the flora benefits, and the other does not benefit, nor is it harmed. COMPETITIVE EXCLUSION (‘Nurmi effect’) is where a population of microflora are introduced into the GIT and compete with resources or sites of attachment or colonization of a pathogen such as Salmonella, Campylobacter etc, or produce products such as volatile fatty acids that limit the invasion and/or proliferation of particular pathogens. PROBIOTIC is a live microbial feed supplement that beneficially affects the host by improving the intestinal microbial balance. At hatch, the chicken gastrointestinal tract is sterile, yet within a few days a large and complex flora becomes established. The organisms are derived from the environment and feed, by the oral route. Earlier studies relied on culture, but molecular techniques, especially ribosomal 16S RNA and/or DNA analysis, allows better definition of the flora. Different studies have shown that the type of organisms in the different segments of the gastrointestinal tract of healthy chickens were similar in various parts of the world, but the relative proportions differed. Overall, each individual bird has its own distinctive GIT microbial flora, and the interaction of dietary composition and the microflora affects intestinal development, mucosal architecture and mucus composition of the GIT. In any segment, exponential growth phase of bacteria is rapidly replaced by retardation due to nutrient depletion, accumulation of inhibitory metabolites, occupation of binding sites, host immune response and/or ingesta flow and wash-out of bacteria. The development of the flora in any segment is impeded by the movement of the ingesta, but in the full caecae, the generational interval may be as short as 40 minutes thus allowing rapid exponential Date printed: 8/02/2016 page 21 of 24 Rod Reece Infectious stunting syndromes of chickens population increase. The early stage post-hatch is a critical period for the establishment of a ‘healthy’ GIT microbial community. There are approximately 500 bacterial species in the gastrointestinal flora of the domestic fowl, with some estimates exceeding 600 species. Viruses, especially bacteriophages, are numerous, and there are also fungi and protozoa. The questions to be asked are: what is the ‘normal’ and/or optimal gastrointestinal flora of the domestic fowl, and how is that obtained? The GIT flora is a complex microbial community that interacts with the host along the entire length of the GIT tract. By 3-4 days post hatch, the flora in the gastrointestinal tract of a chick plateaus at about 1013 organisms with most of these in the caecum (at 1011/gm). At different regions of the GIT there are significantly different physicochemical conditions, nutrient bioavailability, differing host response, and microflora interactions. The bacteria themselves may be located in the lumen, buried in the mucus layer or adherent to the intestinal mucosa. Caecal bacteria produce several B vitamins as well as vitamins E and K, although little is actively absorbed through the wall. The crop flora contains 103-5 bacteria/g and consists of an almost continuous layer of Lactobacilli attached to the epithelium, plus Enterococci (sometimes referred to as ‘lactic acid bacteria’ because they ferment carbohydrate to lactic acid; previously known as Group D Streptococcus), coliforms and yeast. The acidity of the proventriculus and gizzard renders these environments relatively unsuitable for bacterial colonization. In the duodenum, bacterial growth is restricted because of high oxygen tension, chemical inhibition of bacterial growth due to the enzyme-rich milieu and antibacterial substances (bile salts), high competition rate from host nutrient absorption, high passage rate of ingesta into and out of the duodenum (peristaltic and retro-peristaltic), continuous sloughing of epithelial cells into the lumen and mucus production, and immunological defense mechanisms; however, bacteria similar to those found in the crop are present in low and oscillating numbers. The terminal ileum contains 108-9 bacteria/g, predominantly Lactobacilli (70% of the flora in this segment), Enterococci and coliforms. The caecae, largely because of the slow turnover of contents, develops a great diversity and density of 1011 anaerobic bacteria/g, initial populations are often transient and a more stable microflora develops at about 6-8 weeks including gram-negative Bacteroides, gram-positive Bifidobacteria, Lactobacilli, Enterococci, Coliforms, and Clostridiaceae, along with occasional Proteus spp and Pseudomonas aeruginosa. E.coli comprise a low proportion of the GIT flora: about 105 cfu/g in ileum and 107 cfu/g in caecae . Cl perfringens belong to Clostridiaceae Group 1 and are relatively rare in the chicken GIT, but they are susceptible to antibiotics; sensitivity of other bacterial species to antibiotics is more variable. In-feed antibiotics, including polyether ionophorous anticoccidials, alter the dynamics of the GIT microbial community. (see Gabriel, L., Lessire, M. et al 2006. Microflora of the digestive tract: critical factors and consequences for poultry. World’s Poult Sci J 62:409) Gram negative bacteria (although only a small proportion of the GIT flora) release endotoxins derived from the cell wall lipopolysaccharides, when they undergo lysis. Damage to the intestinal tract mucosa by enteric pathogens such as protozoa, bacteria or virus, or by other processes, alters the motility of the intestine, changes the mucous layer characteristics, may or may not breach the epithelial integrity thus allowing invasion of the pathogen or other members of the microbial flora into the deeper tissues of the intestines, and alters the environment (by reducing nutrient absorption or adding excess sloughed cells or fluid exudation) in which the flora live thus facilitating change in microflora population dynamics often referred to a Date printed: 8/02/2016 page 22 of 24 Rod Reece Infectious stunting syndromes of chickens ‘dysbacteriosis’ or ‘dysenterobacteriosis’, although ‘dysenterobiosis’ is our preferred term (to indicate that the alteration may include other organism besides bacteria). This may then create a cascade of significant and detrimental changes by predisposing to overgrowth with .pathogens such as Cl perfringens leading to necrotic enteritis, aggravating already impaired nutrient digestion and absorption, retarding healing and resolution of pre-existing damage, excessive loss of electrolytes and water, and/or further changing intestinal structure and function by changing mucous secretions. Appendix 3 STRUCTURE AND FUNCTION OF THE AVIAN PANCREAS with particular reference to the DOMESTIC FOWL In the domestic fowl, the pancreas consists of a dorsal and ventral lobe, and a smaller splenic lobe. The larger lobes have ducts extending their full length. The exocrine pancreas has lobules of tubulo-acinar glands lined by columnar or triangular acinar epithelial cell with a large basal nucleus. The cytoplasm of the basal third of the cell is dense, finely granular and basophilic, whereas the apical cytoplasm is filled with large eosinophilc zymogen granules containing enzyme precursors. Following feeding, many, but never all, of the granules are released into the acinar lumen via exocytosis. To replenish the cell, the Golgi apparatus swells and produces granules which are derived from the endoplasmic reticulum; these move away towards the apex. The fully replete cell then enters a resting phase awaiting stimulus and discharge. The acini contain neck cells and are connected to ductules lined by low cuboidal cells, which progressively link to wider ducts, lined by a highly folded mucosa with columnar epithelial cells. The thicker ducts have a prominent muscularis and converge onto the hillock of Vater (papilla duodenalis) and discharge into the intestinal lumen at the distal duodenum adjacent to the common bile duct. The ductule cells secrete water and bicarbonate, as well as some mucin. It is common to observe eosinophilic homogenous secretion in the lumen of ductules and ducts. The pancreatic acini are innervated. Lobulation of the chicken pancreas is poor. The organ is enclosed in serosa. The pancreatic secretion rate in domestic fowls is greater than in mammals and less affected by fasting. The secretion contains buffers, especially bicarbonate secreted by the duct lining cells, and enzymes such as trypsinogen, chymotrypsinogen, alpha-amylase, lipase, carboxypeptidase etc produced by the acinar epithelial cells. Some of these enzymes exit the cells as precursors ie enterokinase produced by duodenal mucosal epithelial cells in the intestinal lumen activates trypsinogen to trypsin; trypsin then activates chymotrypsinogen to chymotrypsin. Optimal activity of trypsin occurs at neutral pH so the acidity of the chyme exiting the gizzard into the duodenum has to be neutralized, hence the bicarbonate and other buffers in the pancreatic secretion. The production of pancreatic enzymes is relatively constant per unit of ingesta; but release is pulsatile and dependent upon chyme in the duodenum. The activity of pancreatic enzymes is poorly developed until about 7 days post-hatch. Lipase is the main pancreatic enzyme and is crucial for lipid digestion, but there are troughs and peaks in production of this, and other pancreatic enzymes, during the first few weeks. Synthesis and release of pancreatic secretion is dependent upon neural and endocrine stimuli. In mammals, pancreatic production and release is modified by hormones such as gastrin, secretin (which stimulates duct cells to produce bicarbonate), cholecystokinin, pancreozymin etc, and some of these have been definitely identified in the avian species. Date printed: 8/02/2016 page 23 of 24 Rod Reece Infectious stunting syndromes of chickens The pancreas undergoes rapid post-mortem autolysis, and is also subject to artefacts from rough handling, such as trimming-out with scissors. The duodenal loop with central pancreas lies on the ventral aspect of the abdominal cavity and therefore is a common site of implantation of coelomic cavity tumour metastases or bacterial peritonitis. Foci of extramedullary myelopoiesis are often noted in the trabeculae of young chicks, and lymphoid foci and germinal centres are common in the trabecula of chicken pancreases. The endocrine pancreas exists as scattered small islets of Langerhans, with the islets being more numerous in the splenic lobe. Alpha islets contain columnar alpha cells with coarse granules, arranged as anastomosing cords with a sinusoidal vascular network, and small delta cells with minute basophilic granules, arranged as single cells or small groups. The beta islets contain polygonal beta cells arranged as strands with numerous fine amphophilic granules, as well as a few delta cells. Some alpha cells are also argyrophililic. Alpha cells secrete glucagon; beta cells secrete insulin and amylin; and delta cells secrete somatostatin: there are also a few other types of cells which secrete pancreatic polypeptide etc. Bile salts enter the terminal duodenum via the hillock of Vater and are essential for optimal pancreatic-derived lipase function, and thence digestion and absorption of dietary triglycerides and fatty acids, and also the absorption of fatsoluble vitamins A, D3 and E. Date printed: 8/02/2016 page 24 of 24

![Newsletter 26.04.13[1]](http://s3.studylib.net/store/data/006782410_1-da9f3895022a2272f47db633b66536f9-300x300.png)