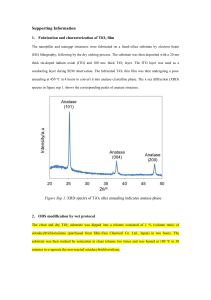
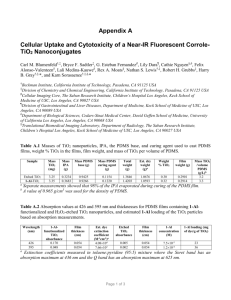
Electronic supplementary information
advertisement

Electronic supplementary information Biocompatible TiO2 Nanoparticle-based cell immunoassay for 5 Circulating Tumor Cells Capture and Identification from Cancer Patients Rongxiang He, Libo Zhao, Yumin Liu, Nangang Zhang, Boran Cheng, Zhaobo He, Bo Cai, Sizhe Li, Wei Liu, Shishang Guo, Yong Chen, Bin Xiong* and Xing-Zhong Zhao* 10 Rongxiang He, Yumin Liu, Nangang Zhang, Zhaobo He, Bo Cai, Sizhe Li, Wei Liu, Shishang Guo, and Xing-Zhong Zhao* Key Laboratory of Artificial Micro- and Nano-structures of Ministry of Education, School of Physics and Technology, Wuhan University, Wuhan, 430072,China. E-mail: xzzhao@whu.edu.cn 15 Boran Cheng and Bin Xiong* Zhongnan Hospital of Wuhan University, Hubei key laboratory of Tumor Biological behaviors, Hubei Cancer Clinical Study Center, Wuhan, ,430071, China. E-mail:binxiong88@yahoo.com Libo Zhao 20 c Beijing National Laboratory for Molecular Sciences, Key Laboratory of Molecular Nanostructures and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, China. Department of Molecular and Medical Pharmacology, University of California Los Angeles (UCLA), 570 Westwood Plaza Building 114, Los Angeles, 90095, California, USA. 25 Yong Chen Department of Chemistry, Ecole Normale Superieure, 24 rue Lhomond, 75231, Paris, France. Fig. S1 Surface modification procedure of the TiO2 nanoparticles on glass substrate with antibody. 5 10 Fig. S2 The thickness (a) and transmittance (b) of TiO2 nanopaticles film fabricated at different slurry concentration. (c) The SEM of the section of the film fabricated when the concentration is 100 mg/mL. The scale bar is 250 nm. 5 Fig. S3 AFM and SEM images of the TiO2 nanoparticles on glass substrate (a) ~ (f) with surface roughness of 36, 51, 60, 77, 85 and 94 nm, respectively. Fig. S4 The FTIR and Raman spectra characterizatioin of the TiO2 nanoparticles after annealing at 500℃ for 15 5 min. Fig. S5 The difference of cell capture performance of the three substrates: TiO2 NPF-Bare (without any modification), TiO2 NPF-SA (substrate modified with streptavidin) and TiO2 NPF-SA-EpCAM (substrate modified with both streptavidin and anti-EpCAM antibody). HCT 116 cells was used. 5 Fig.S6 The illustrate of the microchip integrated with TiO2 nanoparticle in microchannel fabrication. The PDMS with microchannel was reversible bonding with glass substrate. Then the capillary force can drive then TiO2 nanoparticles slurry filled the microchannel. After baked at 70 ℃ for half hour, the PDMS was carefully peeled off from the glass 10 substrate. The substrate with TiO2 nanoparticles on the surface was annealed at 500 ℃ for 15 minutes. Then the substrate was bonded with PDMS by oxygen plasma. Cancer Patients Colorectal Cancer Gastric Cancer Patient No. Male/Female CTC counts (1.0 mL blood sample) Standard TNM Cancer Staging Metastatic #1 Male 4 T4N0M0 - #2 Male 6 T3N0M0 - #3 Male 2 T4N1M0 - #4 Male 7 T4N1M0 - #5 Male 6 T2N1M0 - #6 Female 7 TxNxM1 + #7 Female 3 T4NxM0 - Table S1. The CTCs counts based on TiO2 nanoparticles capturing devices for 1.0 mL colorectal and gastric cancer 5 patients peripheral blood sample. The patients basic information , standard TNM staging, metastatic vs. localized.