4.08 Guided Notes
advertisement
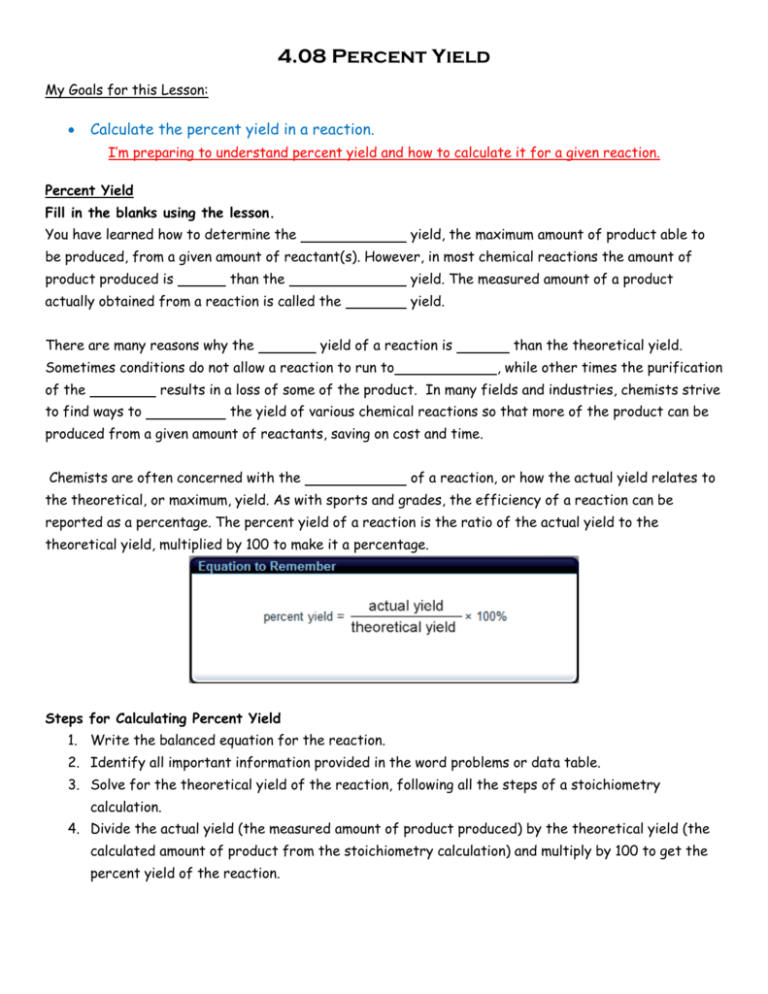
4.08 Percent Yield My Goals for this Lesson: Calculate the percent yield in a reaction. I’m preparing to understand percent yield and how to calculate it for a given reaction. Percent Yield Fill in the blanks using the lesson. You have learned how to determine the yield, the maximum amount of product able to be produced, from a given amount of reactant(s). However, in most chemical reactions the amount of product produced is than the yield. The measured amount of a product actually obtained from a reaction is called the There are many reasons why the yield. yield of a reaction is Sometimes conditions do not allow a reaction to run to of the than the theoretical yield. , while other times the purification results in a loss of some of the product. In many fields and industries, chemists strive to find ways to the yield of various chemical reactions so that more of the product can be produced from a given amount of reactants, saving on cost and time. Chemists are often concerned with the of a reaction, or how the actual yield relates to the theoretical, or maximum, yield. As with sports and grades, the efficiency of a reaction can be reported as a percentage. The percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to make it a percentage. Steps for Calculating Percent Yield 1. Write the balanced equation for the reaction. 2. Identify all important information provided in the word problems or data table. 3. Solve for the theoretical yield of the reaction, following all the steps of a stoichiometry calculation. 4. Divide the actual yield (the measured amount of product produced) by the theoretical yield (the calculated amount of product from the stoichiometry calculation) and multiply by 100 to get the percent yield of the reaction. Complete each example step by step using the tabs in the lesson as a guide. Example One: 9.80 grams of magnesium metal reacts with excess hydrochloric acid, and 29.8 grams of magnesium chloride are produced by the reaction. What is the percent yield of this reaction? Mg + HCl → MgCl2 + H2 1. Write the balanced equation for the reaction. 2. Identify all important information provided in the word problems or data table. 3. Solve for the theoretical yield of the reaction, following all the steps of a stoichiometry calculation. Show all the work, steps and units!! (This is required in the assessments for you to earn full credit.) 4. Divide the actual yield (the measured amount of product produced) by the theoretical yield (the calculated amount of product from the stoichiometry calculation) and multiply by 100 to get the percent yield of the reaction. Show all the work, steps and units!! Example Two: When Mandy allows 38.5 grams of oxygen to react with 4.05 grams of hydrogen gas, she is able to collect 35.3 grams of water. What is the theoretical yield of this reaction, and what is the percent yield? O2 + H2 → H2O 1. Write the balanced equation for the reaction. 2. Identify all important information provided in the word problems or data table. 3. Solve for the theoretical yield of the reaction, following all the steps of a stoichiometry calculation. Show all the work, steps and units!! (This is required in the assessments for you to earn full credit.) 4. Divide the actual yield (the measured amount of product produced) by the theoretical yield (the calculated amount of product from the stoichiometry calculation) and multiply by 100 to get the percent yield of the reaction. Show all the work, steps and units!! Example Three: When 70.0 grams of MnO2 reacted with 128.0 grams of HCl, the reaction resulted in a 62.7 percent yield of chlorine gas. What is the actual yield of chlorine gas in grams? MnO2 + HCI → MnCl2 + H2O + Cl2 1. Write the balanced equation for the reaction. 2. Identify all important information provided in the word problems or data table. 3. Solve for the theoretical yield of chlorine gas. Show all the work, steps and units!! (This is required in the assessments for you to earn full credit.) 4. Divide the actual yield (the measured amount of product produced) by the theoretical yield (the calculated amount of product from the stoichiometry calculation) and multiply by 100 to get the percent yield of the reaction. Show all the work, steps and units!! Be sure to do the practice exercises in the “Let’s Practice!” section of the lesson AND the “practice worksheet” on the Activity page.








