Matter Test Booklet
advertisement

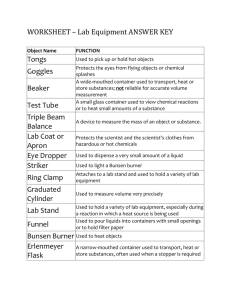
Matter Test This test addresses the following Washington state Science Standards: 6-8 PS2A Substances have characteristic intrinsic properties such as density, solubility, boiling point, and melting point, all of which are independent of the amount of sample. 6-8 PS2B Mixtures are combinations of substances whose chemical properties are preserved. Compounds are substances that are chemically formed and have different physical and chemical properties from the reacting substances. 6-8 PS2C All matter is made of atoms. Matter made of only one type of atom is called an element. 6-8 PS2D Compounds are composed of two or more kinds of atoms, which are bound together in well-defined molecules or arrays. 6-8PS2E Solids, liquids, and gases differ in the motion of individual particles,. In solids, particles are packed in a nearly rigid structure; in liquids, particles move around one another; and in gases, particles move almost independently. 6-8 PS2F When substances within a closed system interact, the total mass of the system remains the same. This concept, called conservation of mass, applies to all physical and chemical changes. The standard that is addressed by each question is identified by a capital letter in parentheses at the end of the question. Directions: Do not write on this test paper. Answer all questions on the answer sheet provided. Density Substance Water (H2O) Ammonia (NH3) Carbon dioxide (CO2) Sucrose (C12H22O11) Benzene (C6H6) Density in g/cm2 1.000 0.862 0.002 1.587 0.877 Melting Point Substance Water (H2O) Ammonia (NH3) Carbon dioxide (CO2) Sucrose (C12H22O11) Benzene (C6H6) 1 Boiling Point in o C 0 -77.73 -78 186 Boiling Point Substance Water (H2O) Ammonia (NH3) Carbon dioxide (CO2) Sucrose (C12H22O11) Benzene (C6H6) Boiling Point in o C 100 -33.34 -57 1860 80.1 Solubility in Water Substance Water (H2O) Ammonia (NH3) Carbon dioxide (CO2) Sucrose (C12H22O11) Benzene (C6H6) Value 702 ml/ml 0.1782 g/100g of water 201.9 g/100g of water 1.8 g/L 5.5 Your teacher gives you a sample of a substance. You are told by your teacher that the substance is one of the five substances listed in the tables above. You test the substance’s density and find that its density is 0.877 g/cm3. What substance did your teacher give you? (A) A. Ammonia B. Benzene C. Carbon dioxide D. Sucrose 2 Your teacher gives you a second substance telling you again that it is one of the five substances listed in the tables above. You note that this substance is solid and when you heat it you discover that it has a melting point of 186oC. What is this substance? (A) A. Benzene B. Carbon dioxide C. Sucrose D. Water 3 Your teacher gives you an unknown substance for a third time. It is still one of the five substances in the tables above. Your testing reveals that the substance has a density of 0.002 g/cm3, a boiling point of -57oC, and a melting point of -78oC. Which of the five substances is this unknown? (A) A. Ammonia B. Carbon dioxide C. Sucrose D. Water 4 One last time your teacher provides you an unknown substance, still one of the five substances listed in the tables. You discover through scientific investigation that the substance’s boiling point is 80.1oC, its melting point is 5.5oC, it has a density of 0.877 g/cm3 and has a solubility in water of 1.8 g/L. Which substance is it? (A) A. Ammonia B. Benzene C. Sucrose D. Water 5 If oxygen is combined with hydrogen, water is formed. Are the properties of hydrogen, oxygen, and water the same? (B) A. No B. Yes 6 Carbon dioxide can be formed by combining carbon with oxygen. How are carbon dioxide, carbon, and oxygen different from one another? (B) 7 Describe the properties of water and the substances that react to form water. Compare the properties of all of these substances. (B) 8 All matter is made of 9 Name a common element. (C) 10 Name an uncommon element. (C) . (C) 11 What are elements made of? (C) 12 What is the relationship between atom, molecules, elements, and compounds? (D) 13 Draw a labeled diagram showing the relationship between atoms, molecules, elements, and compounds? (D) 14 Atoms and molecules are related. (D) A. False B. True 15 Explain how elements and compounds are related. (D) 16 Which of the following is a true statement about the behavior of the different states of matter inside of a container? (E) A. A gas fills up the entire container no matter how big the container is or how much of the gas there is.. B. A gas takes on the shape of the container but only takes up a certain portion of the space in the container. C. A liquid fills up the entire container no matter how big the container is or how much of the liquid there is. D. A solid fills up the entire container no matter how big the container is or how much of the solid there is. 17 Describe how the particles of a gas move. (E) 18 Which of the states of matter has particles that move the most freely? (E) A. Gas B. Liquid C. Solid D. None of these 19 Why do the particles in a solid have a definite shape and volume? (E) 20 What does the law of conservation of mass state? (F) 21 If 4 g of hydrogen and 32 g of oxygen react in a closed container to form water, how much water is formed? (F) A. 0 g B. 4 g C. 32 g D. 36 g 22 If 12 g of carbon react with 32 g of oxygen carbon dioxide is formed. If this reaction takes place in an open container you will find 0 g of carbon dioxide in the container when the reaction is done. Why don’t you find any carbon dioxide in the container in this case? (F) 23 Would the scenario in number 22 above be an example of a chemical change or a physical change? (F) Name: Astronomy Test Answer Sheet 1 2 3 4 5 6 7 8 9 10 11 12 13 Period: 14 15 16 17 18 19 20 21 22 23 Grades PS2A PS2B PS2C PS2D PS2E PS2F