Accuracy, Precision, Sig Fig, Sci Notation Study
advertisement
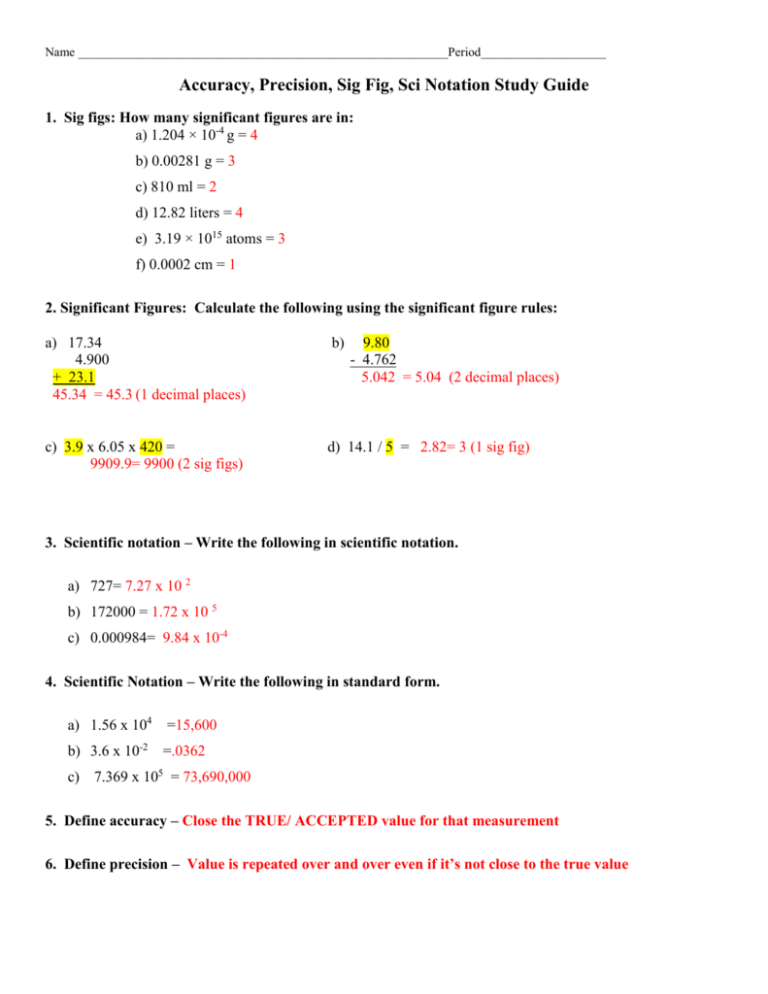
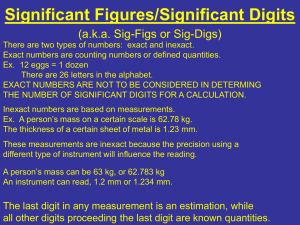
Name ___________________________________________________________Period____________________ Accuracy, Precision, Sig Fig, Sci Notation Study Guide 1. Sig figs: How many significant figures are in: a) 1.204 × 10-4 g = 4 b) 0.00281 g = 3 c) 810 ml = 2 d) 12.82 liters = 4 e) 3.19 × 1015 atoms = 3 f) 0.0002 cm = 1 2. Significant Figures: Calculate the following using the significant figure rules: a) 17.34 4.900 + 23.1 45.34 = 45.3 (1 decimal places) b) 9.80 - 4.762 5.042 = 5.04 (2 decimal places) c) 3.9 x 6.05 x 420 = 9909.9= 9900 (2 sig figs) d) 14.1 / 5 = 2.82= 3 (1 sig fig) 3. Scientific notation – Write the following in scientific notation. a) 727= 7.27 x 10 2 b) 172000 = 1.72 x 10 5 c) 0.000984= 9.84 x 10-4 4. Scientific Notation – Write the following in standard form. a) 1.56 x 104 =15,600 b) 3.6 x 10-2 =.0362 c) 7.369 x 105 = 73,690,000 5. Define accuracy – Close the TRUE/ ACCEPTED value for that measurement 6. Define precision – Value is repeated over and over even if it’s not close to the true value 7. For each of the following, decide the proper measurement using the correct precision: A. 9.5 cm cm B. 9.51 cm (or 9.50 cm) 8. Look at each target and decide whether the “hits” are accurate, precise, both accurate and precise, or neither accurate nor precise: (Note: An accurate “hit” is a bulls eye!) Accurate?: Yes / No Precise?: Yes / No Accurate?: Yes / No Precise?: Yes / No Accurate?: Yes / No Precise?: Yes / No 9. Each of five students used the same ruler to measure the length of the same pencil. These data resulted: 15.33 cm, 15.34 cm, 15.33 cm, 15.33 cm, 15.34 cm. The actual length of the pencil was 15.85 cm. Describe whether accuracy and precision are each good or poor for these measurements. None of the values are accurate because they are not close to the true/accepted value of 15.98 cm. However, they are all precise because they are within .01 cm of each other. The data is precise (repeatable), but not accurate (correct) 10. Read each graduated cylinder below and record the value with the correct precision. a) 54.0 +/- 0.1ml d) 5.52 +/-.01 ml b) 41.5 +/- 0.1ml e) 70.0 +/- .1 ml 11. 11. Put the following in order of smallest to largest Gram Kilogram dekagram Centigram decigram hectogram c) 53.6 +/- 0.1 ml f) 27.1 +/- .1 ml milligram microgram Kilo-Hecto-Deka-gram-deci-centi-milli 13. Lab equipment a. Which is used to measure mass? Balance b. Which is used to measure the volume of a rectangular block? Ruler or meter stick c. How would you find the volume of a pen (irregular solid)? Graduated Cylinder d. How would you find the volume of water? Graduated cylinder 14. A graduated cylinder has lines every 1 ml. Draw a small portion of a graduated cylinder (draw it sideways in the space below) with this precision, using the numbers between 80 ml and 90 ml. Draw liquid on your drawing so that the meniscus reads 84.8 ml. Explain why you can read this measurement as 84.8 ml and not 84 ml and not 84.86 ml. You can only estimate ONE decimal place beyond the markings on your instrument