Limestone, Calcium Carbonate

Rainer Kündig, Christoph Bühler, Heinz Surbeck
Applied Mineralogy and Non-Metallic Resources
Limestone, Calcium Carbonate
IGP/ETHZ HS 2012
651-4097-00
(Modified from Kündig et. al, 1997: p 326.)
Limestone as mineral resource
Essentially, limestone consists of calcite with minor additions of iron and magnesium carbonate, silica, alumina, and other substances.
Reef limestone is often very pure as it lacks clastic material; it can, however, contain high amounts of dolomite. Well-bedded limestone often contains clay minerals, a prerequisite for its transition to marls, or silica, responsible for the sought-after hard rock (siliceous limestone).
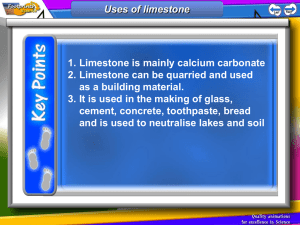
Pure limestone contains 90-95% CaO and is commonly used as burnt lime or products derived therefrom by the building industry. It yields wellhydrating white lime. Highly pure limestone usually finds application in the chemical or iron industry.
Calcareous marls (75-90% CaCO3) and marls (40-75% CaCO3) are the key ingredients of cements, hydraulic binding agents. The composition of cements is highly variable can be adapted to suite specific applications.
Dolomites are defined by a CaO:MgO ratio smaller than 1.7; with increasing CaO content, they pass into calcareous dolomite or dolomitic limestone. Highly pure dolomites (CaO:MgO =
1.39-1.45) are used for the production of metallic magnesium and magnesia cement, but also for the fabrication of glass and the de-acidification of water. Sintered, pure dolomite (CaO:MgO =
1.45-1.70) is used for the lining of cement kilns and smelting furnaces.
The variety of lime, burnt lime, and lime hydrate products has, in conjunction with a myriad of applications, lead to considerable inconsistencies in the nomenclature of raw
(unburned), burnt, and hydrated products. While
Kalk , the German equivalent for lime, is used for both limestone and burnt lime, the English term lime is, in a broad sense used for any of a number of calcium compounds, especially calcium hydroxide, used as an additive to soil or water 1 and, in a more restricted sense for a white caustic alkaline substance consisting of calcium oxide, which is obtained by heating limestone and which combines with water with the production of much heat 2 .
Usage
Since antiquity, limestone, burnt lime, and lime hydrate have been indispensable for numerous uses; there seems to be no limit to the number of applications of this basic substance.
The following branches of industry are mentioned with decreasing economic importance: iron and steel industry, chemical industry, construction
The materials industry, and agriculture. industry, iron and steel industry building
uses lime in the metallurgical process to obtain an alkaline slag, which binds undesirable accompanying elements such as silicon, aluminium, sulphur, and phosphorous. Modern metallurgical processes so
1 The Oxford Dictionary, Oxford University Press, 2012.
2 ibid.
strongly rely on calcium oxide that their operation without them is inconceivable.
The chemical industry offers a very wide range for the application of lime products. Here, lime products either are a component of the end product, e.g., glass, calcium carbide, or calcium cyanamide, or serve as auxiliary agent in the production of other substances, e.g., sugar or soda. Then, the purification of wastewaters from industry and households, which is of vital importance for environmental protection and sustainability, is made possible by the predominant application of lime as a precipitating agent.
The construction material industry uses burnt lime predominantly for the production of calcareous sandstone and lime-bound aerated concrete. These products have become the most important building materials in many countries.
Limestone is also frequently used as aggregate for artificial stones, terrazzo tiles, etc. Since time immemorial, limestone has been the most important raw material in the building industry ; it is needed to produce mortars, plasters, and paints.
Limestone is also used as gravel and filler for road building, and as additive for cement. Burnt lime or its hydrate further serves to stabilise cohesive soils, mainly during construction of simple paths or stabilisation of highly stressed roads.
In agriculture , lime prevents the soil from acidification and leads to a good tilth. Calcium carbonate added to animal food promotes the development of the skeleton.
Please note that raw (unburned), burnt, or hydrated dolomite is just as important as the calcareous products mentioned above, it simply has not been mentioned separately as it is not always possible to clearly delineate the two.
An entry into new markets was achieved by micronized calcium carbonate in the mid 20 th century. As a consequence of the increasingly refined production of synthetics, paint, and paper, the demand for micronized calcium carbonate has skyrocketed and globally active companies that deal with these highly specialized products have sprung up (e.g., Omya). As diverse the product range is, as diverse are the requirements for calcium carbonate as filler; the finest grit sizes and only the purest of substances are used. Today and especially in Europe, the filler trade constitutes over 10% to the total lime turnover.
(Tegethoff, 2001: p. 166.)
References
(Harben, 2002: p. 83.)
Harben, P.W. 2002. The Industrial Minerals HandyBook: a
Guide to Markets, Specification & Prices. Industrial
Mineral Information, Surrey, United Kingdom, 4th edition.
Kündig, R., Mumenthaler, T., Eckhardt, P., Keusen, H.R.,
Schindler, C., Hofmann, F., Vogler, R, Guntli, P. 1997.
Die mineralischen Rohstoffe der Schweiz.
Schweizerische Geotechnische Kommission, ETH
Zurich, Zurich.
Tegethoff, F.W. (Ed) 2001. Calcium Carbonate: from the
Cretaceous Period into the 21st century. Birkhäuser
Verlag, Basel, Switzerland.