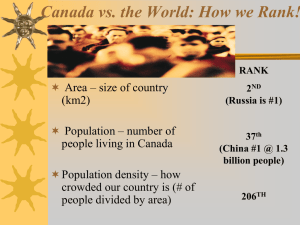
Families - msmcgartland
advertisement

SCH 4C Review: Functional Groups, Organic Families, Properties, and Reactions Functional Groups Functional groups give defining properties to the different families of organic compounds. Many of the functional groups are listed in the Summary of p.201 of your text and can be simply described by how they look, e.g. C = C bond, or a C - O bond, or a C - Cl bond. Other functional groups have a specific name for the atoms and their bonding arrangement. Give the specialized name for each of the following functional groups: Structure Name Structure O Name Structure O Name OH C C OH Families Give the name of each organic family shown. Use your textbook or notes only after you have tried to answer them on your own. Structure C Structure C R Structure O R Family C Family C Structure O C Family Structure OH H Structure O Family Family C Structure O Family C R C R Family C Structure R R C Structure OH Family O Family R’ O R’ Properties a) Rank the molecules from each of the following groups in order from the one with the highest melting point (#1) to the one with the lowest melting point (#3): Group 1: i) CH3CH2CH2CH2CH2 - Br Rank: ____ ii) CH3CH2CH2CH2CH3 Rank: ____ iii) CH3CH2CH2CH2CH2 - OH Rank: ____ Group 2: i) CH3CH2CH2CH2CH2CH2CH3 Rank: ____ ii) CH3CH2CH2CH2CH3 Rank: ____ iii) CH3CH2CH3 Rank: ____ b) Rank the molecules from each of the following groups in order from the one with the greatest solubility in water (#1) to the one with the lowest solubility in water (#3): Group 1: i) CH3CH2CH2CH3 Rank: ____ ii) CH3CH2CH(OH)CH3 Rank: ____ iii) CH3CH(Cl)CH2CH3 Rank: ____ Reactions Show and name the product(s) and balance the equations for each of the following reaction types: a) H CH3C H b) C4H10 + O2 CH + Br2 (complete combustion) (addition) c) d) HC H C - CH3 + F2 H H C C (addition) (addition) e) C3H8 + O2 f) C2H5OH + (incomplete combustion - some of both C and CO) g) CH3CH2COOH + CH3CH2OH H2SO4 esterification (show all bonds) Text Questions p.256 #1, 2, 7, 9 a) - p) H + H2O O2 (complete combustion of ethanol) SCH 4C Review: Functional Groups, Organic Families, Properties, and Reactions Functional Groups Functional groups give defining properties to the different families of organic compounds. Many of the functional groups are listed in the Summary of p.201 of your text and can be simply described by how they look, e.g. C = C bond, or a C - O bond, or a C - Cl bond. Other functional groups have a specific name for the atoms and their bonding arrangement. Give the specialized name for each of the following functional groups: Structu re Name Structure Name Structure O O C C OH Name OH Families Give the name of each organic family shown. Use your textbook or notes only after you have tried to answer them on your own. Structure Family C C Structure O R C H Structure O Structure R Family Family Family OH Structure O C OH Structure O Structure C C Family Structure Family Family C C Structure Family R O R C R Family R’ R C O R’ Properties a) Rank the molecules from each of the following groups in order from the one with the highest melting point (#1) to the one with the lowest melting point (#3): Group 1: i) CH3CH2CH2CH2CH2 - Br Rank: ____ ii) CH3CH2CH2CH2CH3 Rank: ____ iii) CH3CH2CH2CH2CH2 - OH Rank: ____ Group 2: i) CH3CH2CH2CH2CH2CH2CH3 Rank: ____ ii) CH3CH2CH2CH2CH3 Rank: ____ iii) CH3CH2CH3 Rank: ____ b) Rank the molecules from each of the following groups in order from the one with the greatest solubility in water (#1) to the one with the lowest solubility in water (#3): Group 1: i) CH3CH2CH2CH3 Rank: ____ ii) CH3CH2CH(OH)CH3 Rank: ____ iii) CH3CH(Cl)CH2CH3 Rank: ____ Reactions Show and name the product(s) and balance the equations for each of the following reaction types: a) H H CH3C CH + Br2 (addition) b) C4H10 + O2 (complete combustion) c) HC C - CH3 + F2 (addition) d) H H H C C H + H2 O (addition) e) C3H8 + O2 (incomplete combustion - some of both C and CO) f) C2H5OH + O2 (complete combustion of ethanol) g) H2SO4 CH3CH2COOH + CH3CH2OH esterification (show all bonds) Text Questions p.256 #1, 2, 7, 9 a) - p)