Molecular Models Lab
advertisement

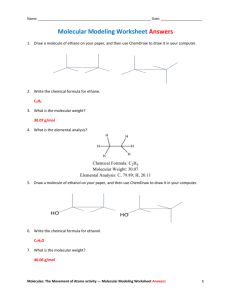
CHEM 3760 Orgo I, S12 Exp 1 (Lab #2) (Molecular Models) LAB REPORT INSTRUCTIONS This report is due at the beginning of next lab and it needs to reflect the student’s own work, no group work for this assignment. Print clearly in your lab notebook so that the carbon copies are legible. Label everything you hand in with your name, date, and experiment number. Individual Write-up (50 pts total) Carefully answer all tasks into your lab notebook. Include the questions / tasks in the report. (1 pts) Date and Title of Experiment (2 pts) Objective (in your own words, 2 sentences) STRUCTURE AND CONFORMATIONAL ANALYSIS USE YOUR MOLECULAR MODEL SET to work on these exercises. Take notes on this worksheet. Then transfer your good answers to your lab notebook. Include descriptions that will help you remember this exercise and each task. Conformational Analysis Atoms and bonds repel each other due to electrostatic repulsion between their electrons. There is strain when the electron clouds of atoms in the molecule are too close to each other; this is known as steric strain. To minimize steric strain, the bonds and atoms in a molecule prefer to be as far away from each other as possible. Conformational analysis is the study of the energy and stability between conformers of a molecule. Conformers are structures of the same molecule that arise as a result of the rotation about a single bond. The dihedral angle (also known as torsion angle) is the angle between two groups as you look down a C-C bond. One useful way to depict conformers is the Newman Projection. The Newman projection looks down the C-C bond as shown in the figure below (left and right), in which the substituents are either staggered or eclipsed. Learn how to correlate Newman projections to your molecular models, and how to convert regular drawings into Newman projections and vice versa. 1 H H H CH3 H H3C H H H H3C H H H H H H H H There are three main types of conformations: Syn (Eclipsed) Cl Cl H H Gauche (Staggered) dihedral angle, H H = 0º dihedral angle between Cl and Cl Anti (Staggered) dihedral angle, Cl H Cl H H Cl H H dihedral angle, H H Cl H = 60º dihedral angle between Cl and Cl = 180º dihedral angle between Cl and Cl A) ETHANE Begin by building a model of ethane (CH3CH3). Look down the C-C bond and rotate the back carbon around the bond in 60° increments, leaving the front carbon of the molecule stationary. The resulting view gives the Newman projections for ethane. 1) Fill in appropriate atoms in the Newman projections for ethane below. (4 pts) A B B) 1) How many unique Newman conformations do you observe? (2 pts) ___________ A molecule also experiences torsional strain due to electrostatic repulsion of the electrons in a bond. Torsional strain is the resistance to rotation about a single bond caused by the eclipsed bond interactions. The figure below shows the potential energy of ethane as the C1-C2 bond in ethane rotates. The y-axis shows the potential energy relative to the most stable conformations as the dihedral angle () increases. 2) Which conformer of ethane (A or B) do you think will be the highest in energy (least stable)? (2 pts) ___________ Explain. 2 3) Which conformer of ethane (A or B) do you think will be the lowest in energy (most stable)? (2 pts) ___________ Explain. 4) In the energy diagram below, label each peak and trough with the letter (A or B) to which it corresponds in your Newman diagrams in question 1 above. (7 pts) E 0 60 120 180 240 300 360 Degrees of Rotation C) BUTANE Next, make a model of butane (CH3CH2CH2CH3). 1) Look down the C2-C3 bond axis. Begin with the methyl groups totally eclipsed and rotate around the C2-C3 bond in 60º increments and complete the Newman Diagrams shown below (leave the front part of the molecule stationary). Label each drawing as anti, gauche, eclipsed (H/CH3 eclipsed), or totally eclipsed (CH3/CH3 eclipsed). (7 pts) A B C D __________ __________ __________ __________ E ___________ F G ___________ __________ (a) Which conformer of butane—anti, gauche, eclipsed, or totally eclipsed—do you expect to be highest in energy (least stable)? Explain. (2 pts) 3 (b) Which conformer of butane—anti, gauche, eclipsed, or totally eclipsed —do you expect to be lowest in energy (most stable)? Explain. (2 pts) (c) In the energy diagram provided below, label each peak and trough with the appropriate letter (A, B, C, etc) that corresponds to the relative energy of the Newman Projection. (7 pts) A A C C E E B D 0 60 120 240 180 300 360 Degrees of Rotation D) CYCLOHEXANE Cyclohexanes widely occur in natural products. A vast number of compounds, including many important pharmaceutical agents, contain one or more cyclohexane ring. Planar cyclohexane does not exist, instead it exists in a chair form. If cyclohexane were planar, all of the H atoms would be eclipsed resulting in both steric strain and torsional strain. In reality, cyclohexane has neither torsional strain nor angle strain. Angle strain is observed when the bond angle deviates from the ideal tetrahedral value (109.5º). Rather, cyclohexane achieves tetrahedral bond angles (omitting steric strain) and a staggered conformation (omitting torsional strain) by assuming an arrangement that chemists call a chair conformation (see below). The chair conformation is the most stable form of all possible cyclohexane conformations. Assemble a model of cyclohexane in the chair conformation and place your model on the bench. Notice how it is supported by three of the axial hydrogen atoms (Ha). The other three axial hydrogen atoms are directed up perpendicular to the bench. Notice also that the equatorial hydrogen (He) atoms are pointing towards the equator of the molecule. Each C-He bond is parallel one of the bonds in the ring. Now, convert your ring into the other possible chair conformation as shown below and practice flipping from one chair form to the other one. From left to right (see below), this is accomplishes by pointing C1 down and C4 up. 1) In the structure to the right below, put a circle around each of the 6-axial hydrogen atoms and a box around each of the 6-equatorial hydrogen atoms. Ha Ha eH Ha He eH H 1 He He 4 He Ha H aH Ha Ha = axial; H e = equatorial 4 chair flip H H H 4 H H H H H H 1 H (a) What happens to the equatorial hydrogen atoms (He) as the ring undergoes a flip? (b) What happens to the axial hydrogen atoms (Ha) as the ring undergoes a flip? 2) Practice drawing both chair conformations of cyclohexane in the space provided below. The chair can be obtained by drawing three sets of parallel lines. 5 6 E) SUBSTITUTED CYCLOHEXANES The hydrogens on cyclohexane do not have to be shown at all times. However, regarding other atoms / groups attached to the cyclohexane ring, it is important to recognize that such groups (=substituents) can assume two conformations – axial or equatorial. For example, the methyl group in methylcyclohexane can be in the equatorial or axial position as shown below. The energy of the conformer with the substituent in the equatorial position is lower in energy and therefore more stable; such a conformer avoids strain. 1) Build a model of cis-4-methoxycyclohexanol. The prefix “cis” refers to both substituents being on the same side. 2) Draw both possible chair conformations. (8 pts) In the first structure show only the groups in the 1- and 4position. Then draw the structure that results from a ring flip. [NOTE: Practice the interconversion of structural representations. Models are very useful to understanding the 3-D aspects of chemical structures, the more you use your models the better you will understand stereochmistry]. 3) Identify the conformer that is more stable. Explain why. (4 pts) Print clearly in your lab notebook so that the carbon copies are legible. Label everything you hand in with your name, date, and experiment number. Submit your report at the beginning of the next lab session. Do not complete the report during your next lab. 7