FTIR and Raman
advertisement

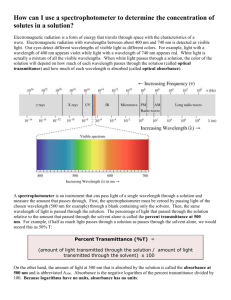
Lab 6 FTIR & Raman Introduction The FTIR is a technique that shines a beam containing many different frequencies of light at once, and measures how much of that beam is absorbed by the sample. It can identify unknown materials, determine the quality of a sample and determine the amount of components in a mixture. The vibrational movement creates a spectrum of wavelength absorbance. Can be used quantitatively to qualitatively. Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system. This instrument can be used for both qualitative and quantitative applications. Microscopy is used to focus an infrared beam on a very specific area of a sample in order to analyze the sample. We used it to measure different fiber samples. Procedure FTIR: 1. Turn on instrument 2. Make sure that the black lever between the FT-IR and the Raman is down 3. Double click on the Varian Resolutions Pro icon in the top right of the display. 4. In the Current scan menu on the bottom of the screen select IR scan. 5. From the Collect menu at the top of the screen select Rapid scan 6. the Electronics tab parameters should be: speed: 20 KHz Filter: 5 UDR: 2 7. the Optics tab parameters should by: IR Source: Mid-IR Beam: internal Detector: DTGS Beamsplitter: KBr Broadband Accessory: none Optical filter: none Aperture: 4 cm-1 at 2000 cm-1 8. click setup. The voltage reading should be between 1-5V. If not, change the selectivity 9. Click background to run a background scan 10. Return to rapid scan from the collect menu and click scan The transformers menu allows you to convert to %T 11. To shut down, close Varian Resolutions Pro and LEAVE EVERYTHING ON Data FTIR We ran different organic solvents mixtures which included Toluene, 2 Propanol, and 2 Butanol in different parts as well as unknowns. Figure 1: Sample of 2-Butanone absorbance spectrum Figure 2: 2 – butanone % transmittance Figure 3: 2-propanol absorbance spectrum. Figure 4: 2-propanol % transmittance. Figure 5: Toluene absorbance spectrum Figure 6: Toluene % transmittance Figure 7: Mix 1 absorbance spectrum Figure 8: Mix 1 % transmittance Figure 9: Mix 2 absorbance spectrum Figure 10: Mix 2 % transmittance Figure 11: Mix 3 absorbance spectrum Figure 12: Mix 3 % transmittance Figure 13: Mix 4 Absorbance spectrum Figure 14: Mix 4 % transmittance Figure 15: Unknown sample absorbance spectrum Figure 16: Unknown sample % transmittance FTIR Microscope We ran different samples of fibers on the instrument. Figure 17: Sample of Cotton Figure 18: Cotton (80%) Polyester (20%) Figure 19: Nylon (85%), Elastane (15%) Figure 20: Polyester (70%), Acrylic (30%) Figure 21: Polyester (50%), Cotton (50%) Figure 22: Unknown sample of fibers. Looked similar to a polyester cotton mix. Conclusions We were able to easily run these instruments. Using the FTIR we were able to run different samples of organic solvents. We obtained good spectra of 2-Propanol, 2-Butanone, and Toluene and the mixtures of each. The unknown sample also had similar peaks to these samples. The instrument was fairly easy to use once we knew where to insert the sample. The program was also easy to operate and we obtained both absorbance and transmittance graphs for both. The FTIR microscope was also easy to use. I am more familiar with microscopes because of biology classes to I enjoyed this portion of the lab. We were able to obtain data to show % transmittance of the fibers we placed under the microscopes. It was weird not getting a picture as our data from a microscope but rather a spectrum graph.