Electronic supplementary material
advertisement

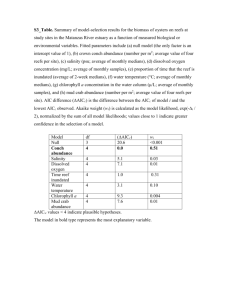
Appendix 1 Telomere analysis. We used the telomere restriction fragment assay, a modified Southern blot that visualizes terminal telomere repeat densities at all molecular weights (see figure below). We calculated the average telomere length in the entire smear, according to a protocol detailed in Salomons et al. [1] and Haussmann and Mauck [2]. Briefly, 5 µL of packed red blood cells, stored frozen in a glycerol buffer, were extracted into agarose plugs using a kit (CHEF Genomic DNA Plug Kit, Bio-Rad). Plugs were digested with a mixture of 3 U Hinf I, 15 U Hae III, and 40 U Rsa I (Roche Applied Science) in a restriction enzyme buffer (NEBuffer 2, New England Biolabs). Half plugs were separated using pulsed-field gel electrophoresis (CHEF Mapper, Bio-Rad) on a 0.8% agarose gel. Run parameters were 21 hours at 3 V/cm and 0.5 (initial) to 7 (final) s switch times. The circulating 0.5x TBE buffer was kept at 14 °C. Gels were dried and then hybridized overnight at 37 °C with 3,000,000 cpm of the telomere-specific 32P-labeled oligo (CCCTAA)4. After hybridization, rinsing and visualization followed Haussmann and Mauck [2]. ImageJ software (version 1.46r) was used to extract telomere smear densities. Lane-specific background was subtracted from each density value. Lower and upper cut-off followed Salomons et al. [1]. Samples were analyzed in pairs (i.e. the 2011 sample of an individual next to its 2012 sample), in random order on four gels, and control samples (from a large sample of a standard individual used in all our lab's analyses) were run twice in each gel to determine inter- (8.7%) and intra-assay variability (<3.6%). Typical gel with telomere smears of black-legged kittiwakes. Telomeres were assayed with the telomere restriction fragment method. Repeated samples of an individual (2011, 2012) were arranged on the gel side-by-side. The molecular weight marker is the central line of the gel. Two control samples (i.e. lines 7 & 23, counting from the left) were run on each gel to control for inter- and intra-gel variations. Appendix 2 rank 1 2 3 4 5 6 7 8 9 10 11 Results of model selection, sorted by model rank, for effects of wintering time (days spent south of 70ºN), stress treatment during reproduction (stress hormone versus control implant), and sex (male versus female) on change in telomere length (%) in black-legged kittiwakes. AICc = Akaike Information Criterion value for finite sample size, ∆AICc = difference in AICc value between this and the model with lowest AICc value, wi = AICc weights, i.e. ratio of AICc values for this relative to the whole set of models. wintering wintering time x time x wintering stress stress wintering stress stress time x treatment x treatment x intercept time treatment sex treatment sex sex sex AICc ∆AICc x x x x 81.21 0.00 x x x 81.91 0.70 x x x x x 84.99 3.78 x x x x x 87.37 6.16 x x 88.29 7.08 x x x 89.03 7.82 x x 94.73 13.52 x x x 94.87 13.66 x x 95.05 13.84 x 95.44 14.23 x x x x x x x x 117.78 36.57 Results of model averaging for models with ∆AICc < 2. Time spent at the wintering grounds was scaled before averaging. model term model-averaged estimate standard error 95% confidence intervals intercept -0.050 1.10 -2.21, 2.11 wintering time 3.56 0.88 1.85, 5.28 stress treatment -4.38 1.19 -6.71, -2.05 sex -3.55 1.57 -6.64, -0.46 wi 0.512 0.360 0.077 0.024 0.015 0.010 0.001 0.001 0.001 <0.001 <0.001 Appendix 3 Telomere loss in kittiwakes did not appear to reflect an increase in their oxidative stress levels (as reflected in titers of reactive oxygen metabolites in plasma) in response to corticosterone treatment. Thus, our empirical evidence currently does not support the hypothesized direct link between the adrenocortical and oxidative stress [3]. We obtained plasma samples for the analysis of oxidative damage immediately before and after the implantation experiment. Samples were kept on ice in the field and stored at -80 ºC within hours, until further analysis. We measured oxidative damage through levels of reactive oxygen metabolites using a kit (d-ROM, Diacron International), and following a protocol for bird plasma [4]. Briefly, 10 µL of plasma were diluted into 200 µL of chromogen-buffer-solution, and incubated at 37 ºC for 75 min. Absorbance was measured at 490 nm using a plate reader (SpectraMax Plus384, Molecular Devices) and converted to concentrations (Carratelli Units, CARR U), using the provided calibrator and the formula CARR U = absorbance sample/absorbance calibrator × concentration of calibrator. All samples were run in duplicate. Inter-plate and intra-plate CV were calculated using the kit standard, and were 1.9% and 2.5%, respectively. We observed no effect of glucocorticoid treatment on either total levels of oxidative damage after implantation (F1,16 = 0.008, p = 0.931) or the change in oxidative damage from before to after implantation (F1,15 = 0.096, p = 0.761). Appendix 4 Results for the effects of migration timing on changes in telomere length (%). Timing of autumn departure rather than spring arrival—defined as the date on which individuals crossed latitude 70ºN south- and northbound, respectively—correlated with changes in telomere length. A later departure was associated with higher telomere loss. The effect did not differ between stress treatments, as indicated by the non-significant interaction between autumn departure and treatment. These results suggest that autumn rather than spring conditions may be most influential on telomere dynamics in kittiwakes. examined event autumn departure spring arrival explanatory terms autumn departure (date crossing 70ºN) stress treatment autumn departure × stress treatment spring arrival (date crossing 70ºN) stress treatment spring arrival × stress treatment estimate ± s.e. -0.381 ± 0.143 -2.262 ± 6.022 -0.082 ± 0.203 0.325 ± 0.230 -3.750 ± 5.748 0.028 ± 0.325 F(d.f.) 9.058(1,11) 13.117(1,11) 0.164(1,11) 1.816(1,10) 3.101(1,10) 0.008(1,10) p 0.012 0.004 0.693 0.208 0.109 0.933 Appendix 5 Raw data animal id 6146199 6146538 6146542 6202402 6217946 6217949 6223753 6223908 6223910 6223911 6223914 6223916 6223917 6223918 stress treatment corticosterone corticosterone control corticosterone control corticosterone control corticosterone control corticosterone corticosterone control control corticosterone wintering time (days) 157 174 189 198 169 166 174 179 153 173 178 165 168 180 sex m m m f m m f f f f f f f f telomere length 2011 (basepairs) 6317.56 8630.85 7834.69 8203.76 7128.48 9805.32 9508.99 8314.11 7084.67 8211.09 9442.68 7631.93 7702.72 6951.70 telomere length 2012 (basepairs) 5456.09 8277.21 8138.01 8321.38 7284.49 8892.12 9681.73 8382.95 6912.34 7886.43 9370.51 7636.39 7936.40 7304.86 References for the electronic supplementary material 1. Salomons H.M., Mulder G.A., van de Zande L., Haussmann M.F., Linskens M.H.K., Verhulst S. 2009 Telomere shortening and survival in free-living corvids. Proc R Soc B 276(1670), 3157-3165. (doi:10.1098/Rspb.2009.0517). 2. Haussmann M.F., Mauck R.A. 2008 New strategies for telomere-based age estimation. Mol Ecol Resour 8(2), 264-274. (doi:10.1111/j.1471-8286.2007.01973.x). 3. Costantini D., Marasco V., Moller A.P. 2011 A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B 181(4), 447-456. (doi:10.1007/S00360011-0566-2). 4. Haussmann M.F., Longenecker A.S., Marchetto N.M., Juliano S.A., Bowden R.M. 2012 Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proceedings of the Royal Society B-Biological Sciences 279(1732), 1447-1456. (doi:10.1098/Rspb.2011.1913).