4.5 metallic bonding
advertisement

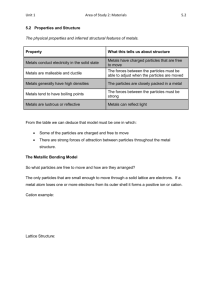
4.5 METALLIC BONDING https://www.youtube.com/watch?v=Bjf9gMDP47s (Metallic Bonding and Metallic Properties) INTERNATIONAL-MINDEDNESS -“The availability of metal resources, and the means to extract them, varies greatly in different countries, and is a factor in determining national wealth. As technologies develop, the demands for different metals change and careful strategies are needed to manage the supply of these finite resources.” DESCRIPTION OF METALS AND METALLIC BONDING: *The structure of a metal is a regular giant lattice that consists of _______________ ions (cations) surrounded by a “sea” of ______________ electrons. Delocalized electrons are not associated with a particular nucleus of a metal, but instead are free to move throughout the entire crystalline lattice forming a “sea” of ____________ electrons. *A metallic bond is the electrostatic attraction between a lattice of positive ions (cations) and delocalized electrons. *The strength of the metallic bond depends on the ____________ of the ions and the ___________ of the metal ion. *The properties of metals are different from ionic and covalent substances due to the formation of non-directional bonds with a “sea” of delocalized electrons. A non-directional bond is one without any defined direction; that is, they act in every direction about the fixed immobile cations. In contrast, a covalent bond is directional; that is, the bonding electron pair must be between the 2 bonding nuclei. In this sense, the position of the bonding electron pair decides the direction of the covalent bonding. SOME IMPORTANT PROPERTIES OF METALS: PROPERTY EXPLANATION Electrical Conductivity *Due to presence of mobile ______________ electrons. Note that metals can conduct in both the __________ and _____________ state. Malleability *Malleability is the ability of a solid to be pounded or hammered into a ___________ or other shape _______________ breaking. Due to the non-directional nature of the metallic bond, cations can slide past one another without disrupting the metallic bond. EXPLANATION OF TRENDS IN MELTING POINTS OF METALS (limit to s and p block elements) *Most metals have quite high melting points. Some exceptions include: ______________ (liquid and room temperature) and the _______________ metals (all melt below 181°C). Consider the following: Alkali Metal Li Na K Rb Cs Charge +1 +1 +1 +1 +1 Ionic Radius (pm) 76 102 138 152 167 Melting Point (°C) 180.5 97.8 63.5 39.3 28.5 *Describe and Explain the Trend in Melting Point Down Group The melting points ______________ down the group because as the ionic radius get ___________, the forces of attraction between them ___ (i.e. the strength of the metallic bond ___). Consider the following: Metal Potassium Calcium Delocalized Electrons per Atom 1 2 Charge of Cation +1 +2 Ionic Radius (pm) Melting Point (°C) 138 100 63.5 842 *Describe and Explain the Trend in Melting Point of the two Metals: The melting point of calcium is significantly higher than potassium. The electrostatic attraction between the cations and the delocalized electrons (i.e. the strength of the metallic bond) is greater in calcium due the greater number of delocalized electrons, ___________ charge and _____________ size of the cation. ALLOYS Alloys are produced by adding one metal element to another metal elements (or ______________) in the ___________ state, so that different atoms can mix; an alloy is a metallic solid ________________held together by _______________ bonding. Alloys usually have enhanced properties (such as greater strength, magnetic properties, ductility, hardness and durability) that are distinct from their component elements due to the different packing of cations in the lattice. The alloy is often more chemically stable, and also often stronger and more resistance to corrosion. Alloys tend to be much stronger due to the presence of different atoms – these disturb the regular lattice and hinder movement of positive ions past one another. The production of alloys is possible because of the non-directional nature of the delocalized electrons, and the fact that the lattice can accommodate ions of different sizes. Examples: ALLOY *Brass COMPOSITION (Principal metal + added metal) Copper + zinc PROPERTIES AND USES *Bronze Copper + tin -plumbing fittings, door handles, window fittings, screws -coins, medals, tools, heavy gears *Steel Iron with carbon (and other elements) -used as structural material (e.g. bridges, buildings) -high tensile strength but corrodes *Stainless steel Iron with other elements such as nickel and chromium -widely used in domestic an industrial appliances due to strength and corrosion resistance *Sterling silver Silver and copper -jewelry, art objects *Solder Lead + tin *Nichrome Nickel and copper -low melting point, used in joining two metals together, especially in electric circuitry -heating elements in toasters, electrical heaters *Duralumin Aluminum, copper and manganese *Pewter Tin, antimony and copper -aircraft, boats, and machinery due to high strength and resistance to corrosion Decorative objects *Dental amalgam Mercury + tin, silver, gold or sodium -teeth fillings