tpj12501-sup-0007-Legends
advertisement

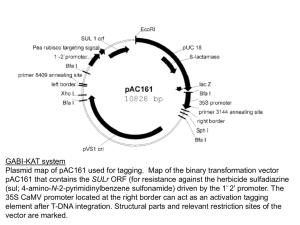
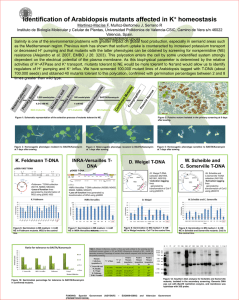
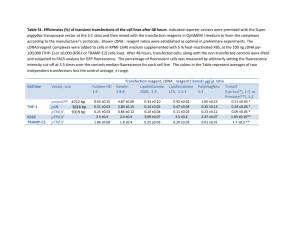
SUPPORTING INFORMATION Figure S1. Alignment of the amino-acid sequence from AtNHD1 with its homolog NHAD from Mesembryanthemum crystallinum. Proteins were aligned using the ClustalW program of the European Bioinformatics Institute (www.ebi.ac.uk). Identical amino-acids are shaded in black, similar amino-acids in grey. Both proteins share 77% idintity and 86% similarity. The predicted plastidic N-terminal platid transit peptides are indicated by black squares. Target sequences were identified using the ChloroP 1.1 Server (http://www.cbs.dtu.dk/services/ChloroP/) Figure S2. Subcellular localization of NHD1-GFP-fusion and TAP38-RFP-fusion proteins. Cotransformation of isolated tobacco protoplasts with the NHD1-GFP-fusion construct and the TAP38-RFP-fusion construct does not indicate a colocalization between NHD1-GFP and the thylakoid located TAP38-RFP fluorescence. (a) NHD1-GFP fluorescence, (b) NHD1-GFP fluorescence, (b) TAP38-RFP fluorescence, (c) Scatter plot of GFP and RFP colocalization according to Pearson‘s correlation. Threshold corresponds to 30%, the colocalization rate is 6,82%. Figure S3. Growth of the E.coli knockout strain KNabc after transformation with empty pTAC-MAT-Tag1 vector or NHD1::pTAC-MAT-Tag1. KNabc cells harbouring empty pTAC-MAT-Tag1 vector (a) or NHD1::pTAC-MAT-Tag1 (b) were grown in LBK medium. Medium was supplemented either with 200 mM KCl (black) as a control or 200 mM NaCl (grey). (a) and( b) Cell grwoth was monitored at OD600. (c) Growth is shown as percentage of the maximum reached growth. Data were fitted to the Boltzman equation and represent the mean of three individual experiments, each with 3 technical replicates. Error bars are ±SE. Figure S4. Characterization of nhd1 mutant plants. (a) Localization of T-DNA insertions in At3g19490 locus. The insertion mutants Salk008491 and Sail107_F07 obtained from ABRC and generated Salk008491x Sail107_F07 mutants were used for T-DNA screening. Arrows represent primer binding sites used for molecular characterization. (b) Phenotype of 5 week old Wt, homozygous Salk008491, heterozygous Sail107_F07 and Salk008491xSail107_F07 plants. (c) Identification of homozygous and heterozygous nhd1 T-DNA insertion mutants via PCR. The primer combination Salk008491_for/Salk008491_rev leads to NHD1 product in Wt (1, 9). The primer combination Salk008491_for/LB335 was used to identify T-DNA insertion (4, 15). The primer combination Sail107_F07_for/Sail107_F07_rev lead to NHD1 product in Wt and heterozygous mutants (5, 7, 10). For identification of T-DNA insertion primer combination LB3/Sail107_F07_rev was used (8, 16). (d) Detection of NHD1 cDNA in T-DNA insertion lines and Wt plants. With the primer combination nhd1_RT_for/nhd1_RT_rev, cDNA of 234 bps was amplified. (e) Diagram of the AtNHD1 construct used for complementation of nhd1-1 mutants. NHD1 cDNA was cloned under the control of the UBQ10 promoter and fused with VENUS. (f) Phenotype of 5 week old Wt and nhd1-1::UBQ10:NHD1 mutants. (g) NHD1 transcript level in complemented nhd1-1 mutants. PCR was performed on cDNA of Wt and nhd1-1::UBQ10:NHD1 plants using the primer combination nhd1_RT_for/nhd1_RT_rev. (h) NHD1-VENUS fusion fluorescence in isolated protoplasts of nhd1-1::UBQ10:NHD1 mutants indicates plastidic NHD1 localization in complemented nhd1-1 plants. Venus fluorescence is shown in green. (A) Venus fluorescence, (B) chlorophyll autofluorescence, (C) merge of venus and chlorophyll autofluorescence. Table S1. Amino-acid quantification in non-stressed and salt stressed Wt, nhd1 knockout and nhd1-1::UBQ10:NHD1 plants. Four week old Arabidopsis plants grown on sand were either watered with or without 150 mM NaCl for three days. Rosettes were harvested and amino-acid levels were quantified. Table S2. Oligonucleotides used for genotyping of nhd1 T-DNA insertion lines, complementation of nhd1-1 with AtNHD1 and for qRT-PCR analysis. Restriction sites are displayed in bolt.