Lab 2: Molar Mass of a Gas
advertisement
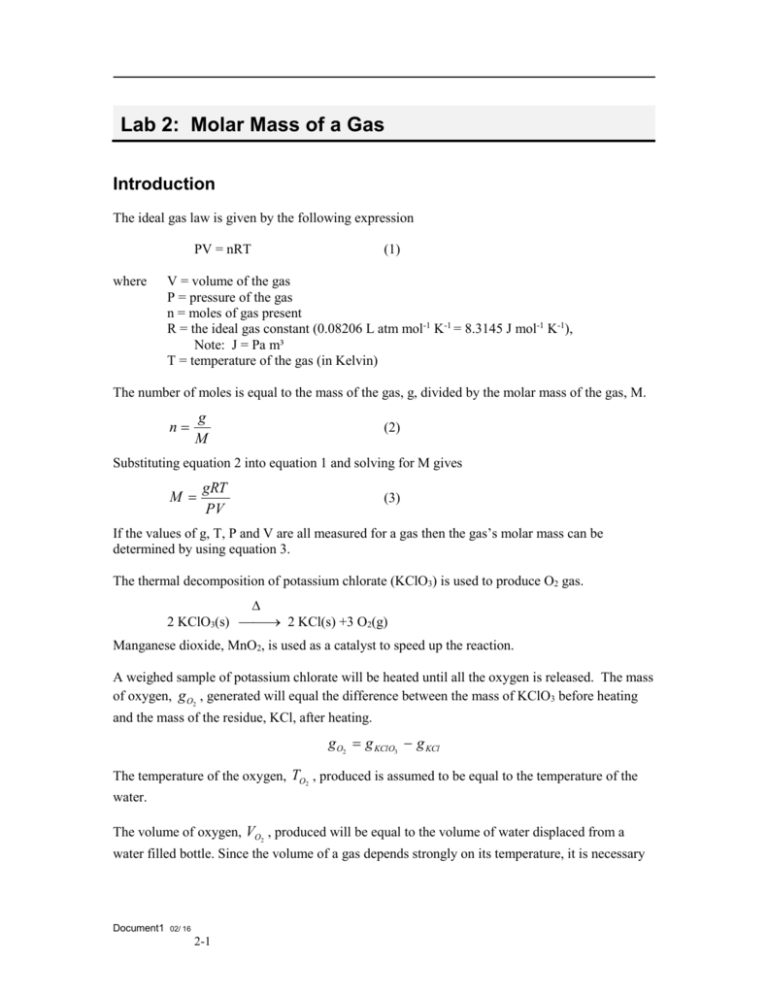
Lab 2: Molar Mass of a Gas Introduction The ideal gas law is given by the following expression PV = nRT where (1) V = volume of the gas P = pressure of the gas n = moles of gas present R = the ideal gas constant (0.08206 L atm mol-1 K-1 = 8.3145 J mol-1 K-1), Note: J = Pa m³ T = temperature of the gas (in Kelvin) The number of moles is equal to the mass of the gas, g, divided by the molar mass of the gas, M. n g M (2) Substituting equation 2 into equation 1 and solving for M gives M gRT PV (3) If the values of g, T, P and V are all measured for a gas then the gas’s molar mass can be determined by using equation 3. The thermal decomposition of potassium chlorate (KClO3) is used to produce O2 gas. 2 KClO3(s) 2 KCl(s) +3 O2(g) Manganese dioxide, MnO2, is used as a catalyst to speed up the reaction. A weighed sample of potassium chlorate will be heated until all the oxygen is released. The mass of oxygen, g O2 , generated will equal the difference between the mass of KClO3 before heating and the mass of the residue, KCl, after heating. g O2 g KClO3 g KCl The temperature of the oxygen, TO2 , produced is assumed to be equal to the temperature of the water. The volume of oxygen, VO2 , produced will be equal to the volume of water displaced from a water filled bottle. Since the volume of a gas depends strongly on its temperature, it is necessary Document1 02/ 16 2-1 to allow the setup to cool to room temperature before the volume measurement is made. The volume of displaced water is determined by measuring the mass of water displaced, g H 2O . g H 2 O gbeaker H 2 O gbeaker Using the density, d H 2O , of water, the volume of water can be calculated. The density of water at various temperatures can be found in the CRC Handbook. VO2 VH 2O g H 2O d H 2O When the water levels in the flask and beaker are the same, then the pressure inside the flask is equal to the barometric pressure of the room. The pressure in the flask is equal to the sum of the pressure due to the oxygen and the vapor pressure of water, Proom PO2 PH 2O . Rearranging gives: PO2 Proom PH 2O The vapor pressure of water at various temperatures can be found in the CRC Handbook. Using these values for g O2 , TO2 , PO2 and VO2 along with the ideal gas constant R in equation 3, the molar mass of oxygen is calculated. Procedure 1. Place the test tube in a small beaker and weigh them. 2. Place approximately 1.0 grams of the potassium chlorate/manganese dioxide mixture into the test tube. 3. Accurately weigh the test tube containing the potassium chlorate mixture and beaker. Record the weight. 4. Nearly fill a 500 mL flask with water. 5. Put about 200 mL of water into a 400 mL beaker. Document1 02/ 16 2-2 6. Setup the apparatus as shown in figure 1. Tube A Pinch Clamp Tube B 7. Figure 1 Place a rubber bulb onto tube A. With the pinch clamp open slowly squeeze on the bulb to fill the tubes connecting the flask with the beaker. There should be no air bubbles in this tube. 8. Tightly fit the stopper with tube A into the test tube containing the potassium chlorate. 9. Adjust the height of the beaker until the water in the flask is level with the water in the beaker (figure 2). Close the pinch clamp. Empty the beaker. This sets the pressure inside the flask equal to the room’s pressure. Pinch Clamp - - Level - KClO3 Figure 2 Document1 02/ 16 2-3 10. Place tube B back into the dry beaker and open the pinch clamp. 11. Gently heat the test tube with a Bunsen burner. Heat slowly first and more vigorously later. Continue heating until no more water is going into the beaker. 12. Allow the test tube and setup to cool to room temperature. Keep tube B submerged into the beaker of water 13. Adjust the height of the beaker until the water in the flask is level with the water in the beaker. Close the pinch clamp. 14. Weigh the beaker and water. Record the weight. 15. Empty out the water and weigh the beaker. Record the weight. 16. Disconnect the apparatus and measure the temperature of the water in the flask. Record the temperature. 17. Weigh the test tube and remaining solid in the same beaker as in step 3. Record the weight. 18. Record the atmospheric pressure. 19. Lookup and record the density and vapour pressure of water. Document1 02/ 16 2-4 Lab 2: Molar Mass of a Gas Date: _______________ Name: __________________________ Partner: _____________ Purpose: Data and Results Mass of test tube + beaker (g) Mass of test tube + beaker + KClO3 before heating (g) Mass of test tube + beaker + residue after heating (g) Calculate the mass of oxygen generated (g) Mass of beaker + water (g) Mass of beaker (g) Temperature of water (°C) Density of water Calculate the temperature of oxygen (K). Document1 02/ 16 2-5 Calculate the volume of oxygen generated. Barometric pressure Vapour pressure of water Calculate the pressure of oxygen. Calculate the molar mass of oxygen (g/mol). Calculate the % error in the molar mass. % 𝐸𝑟𝑟𝑜𝑟 = Document1 𝑀𝑒𝑎𝑠𝑢𝑟𝑒𝑑 𝑉𝑎𝑙𝑢𝑒−𝑇𝑟𝑢𝑒 𝑉𝑎𝑙𝑢𝑒 𝑇𝑟𝑢𝑒 𝑉𝑎𝑙𝑢𝑒 02/ 16 2-6 𝑥 100% Questions: 1. What effect would a loss of O2 through a leak in the tubing have on the experimentally determined molar mass? Explain. 2. Why is it necessary to allow the test tube to cool to room temperature before proceeding with step 12? If this is not done, how would it affect the experimentally determined molar mass? 3. What effect would incomplete decomposition of the KClO3 have on the experimentally determined molar mass? Explain. Conclusions: Document1 02/ 16 2-7







