DRAFT of Multiplexing Protocol
advertisement

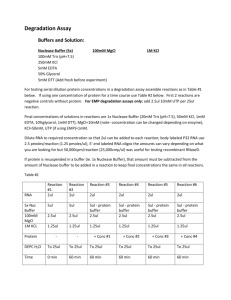
I. Alpha-Enolase Assay Design a. Sample Loading i. Prepare standard dilutions of alpha-enolase diluted in DPBS tween 0.05% solution. 1. Dilutions should be created for 1000ng/mL, 750 ng/mL, 500 ng/mL, 250 ng/mL, 50 ng/mL, 25 ng/mL, 10 ng/mL, 0 ng/mL ii. Add each standard to a different well filled with 25uL of alphaenolase monoclonal antibody beads. iii. Incubate overnight at 25C with shaking b. Biotinylated Polyclonal Antibody i. Magnetize and wash each well with 200uL DPBS tween 0.05%. Perform this step a total of two times. ii. Make sure there is no liquid in the plate, and add 25uL of biotinylated polyclonal antibody @ 5ug/mL in DPBS to the well. iii. Incubate @ room temperature for 2.5 hours c. Addition of Streptavidin PE i. Magnetize and wash each well with 200uL DPBS tween 0.05%. Perform this step a total of two times. ii. Add 25uL of Streptavidin PE (2ug/mL) to each well with no liquid in it (Stock of Streptavidin PE is 2ug/mL and diluted in 1:500 in staining buffer [1x PBS, 1% BSA, pH 7.4]) iii. Incubation with shaking @ 25C for 30 minutes iv. Magnetize and wash each well with 200uL DPBS tween 0.05%. Perform this step a total of two times. v. Add 150uL DPBS with tween 0.05% and shake for an additional 5 minutes vi. Read assay I. II. III. Preparation of Beads for Multiplexing a. Sonicate antibody beads (@ 20X stock solution) for 30 seconds and vortex for 1 minute. b. Add 150uL from each antibody-bead vial to the mixing bottle and bring final volume to 3.0 mL with bead diluent. Vortex the mixed beads Preparation of Quality Controls a. Reconstitute quality controls 1 and 2 with 250uL of deionized water. Invert to mix and briefly vortex. b. Allow vial to sit for 10 minutes c. Transfer controls to labeled polypropylene microfuge tubes. Aliquot and store at -80C for up to one month. Preparation of Wash Buffer a. Bring the 10X Wash Buffer to room temperature and mix to bring all salts into solution. Dilute 60 mL of 10X Wash Buffer with 540 mL deionized water. Store unused portion at 2-8°C for up to one month. IV. Preparation of Beta-2-Microglobulin Standards a. Prior to use, reconstitute the Human Kidney Toxicity Panel Standard with 250 μL deionized water (refer to table below for analyte concentrations). Invert the vial several times to mix. Vortex the vial for 10 seconds. Allow the vial to sit for 5-10 minutes and then transfer the standard to an appropriately labeled polypropylene microfuge tube. This will be used as Standard 6; the unused portion may be stored at ≤ -20°C for up to one month. b. Label five polypropylene microfuge tubes Standard 5, Standard 4, Standard 3, Standard 2 and Standard 1. Add 120 μL of Assay Buffer to each of the five tubes. Prepare serial dilutions by adding 40 μL of the reconstituted standard (Standard 6) to the Standard 5 tube, mix well and transfer 40 μL of the Standard 5 to the Standard 4 tube, mix well and transfer 40 μL of the Standard 4 to the Standard 3 tube, mix well and transfer 40 μL of the Standard 3 to the Standard 2 tube, mix well and transfer 40 μL of the Standard 2 to the Standard 1 tube and mix well. The 0 pg/mL Standard (Background) will be Assay Buffer. V. VI. VII. VIII. Bead Assay (Assay Buffer) – Blocking Step? a. Add 200uL of Assay Buffer to each well. Seal and mix on plate shaker for 10 minutes @ room temperature. b. Decant assay buffer and tap on towel to ensure removal of liquid. Adding samples a. Add 25uL of assay buffer to background wells. b. Add 25uL of each standard or control to appropriate wells. c. Add 25uL of assay buffer to background, standard, and control wells. d. Add 25uL of assay buffer to sample wells. e. Add 25uL of sample to each well. Add Beads to Well a. Add 25uL of beads to each well. Plate on shaker at room temperature overnight Detection Antibody and Strep PE a. Gently remove well contents and wash plate 2 times b. Add 50 μL of Detection Antibodies into each well. (Note: Allow the Detection Antibodies to warm to room temperature prior to addition.) [25uL for 36k panel, modify?] c. .Seal, cover with foil, and incubate with agitation on a plate shaker for 1 hour at room temperature (20- 25°C). DO NOT ASPIRATE AFTER INCUBATION. d. Add 50 μL Streptavidin-Phycoerythrin to each well containing the 50 μL of Detection Antibodies. .) [25uL for 36k panel, modify?] e. f. IX. Seal, cover with foil and incubate with agitation on a plate shaker for 30 minutes at room temperature (20- 25°C). g. Gently remove well contents and wash plate 2 times following instructions listed in the PLATE WASHING section. Final Step before Readout a. Add 100 μL of Sheath Fluid