Electron Dot Diagram Periodic Table Worksheet & Key
advertisement
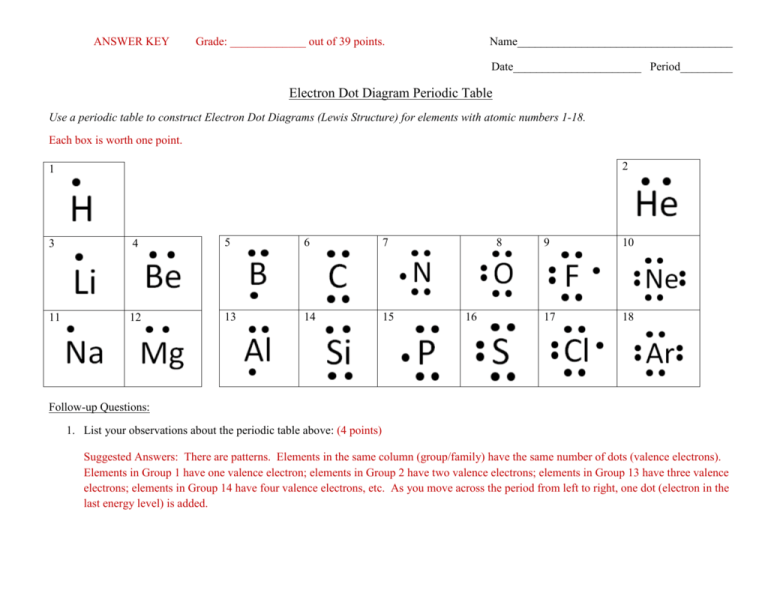
ANSWER KEY Grade: _____________ out of 39 points. Name_____________________________________ Date______________________ Period_________ Electron Dot Diagram Periodic Table Use a periodic table to construct Electron Dot Diagrams (Lewis Structure) for elements with atomic numbers 1-18. Each box is worth one point. 2 1 3 4 5 6 7 11 12 13 14 15 8 16 9 10 17 18 Follow-up Questions: 1. List your observations about the periodic table above: (4 points) Suggested Answers: There are patterns. Elements in the same column (group/family) have the same number of dots (valence electrons). Elements in Group 1 have one valence electron; elements in Group 2 have two valence electrons; elements in Group 13 have three valence electrons; elements in Group 14 have four valence electrons, etc. As you move across the period from left to right, one dot (electron in the last energy level) is added. 2. What do the dots on the Electron Dot Diagram represent? (1 point) The dots represent valence electrons. The dots represent electrons in the last energy level. 3. What can you conclude about the atoms of elements in the same group/family? (4 points) Elements in the same column (group/family) have the same number of dots (valence electrons). Elements in Group 1 have one valence electron; elements in Group 2 have two valence electrons; elements in Group 13 have three valence electrons; elements in Group 14 have four valence electrons, etc. 4. Looking at the periodic table you created, what do you notice about the atoms of elements in the same period? (4 points) As you move across a period from left to right, one dot (electron in the last energy level) is added. 5. Valence electrons, electrons in the last energy level, determine an element’s chemical properties and how it bonds to other atoms. Given this information, what can you conclude about the elements on the periodic table you created? (4 points) Suggested Answer: Atoms of elements in the same group have the same number of valence electrons. Since the atoms of elements in group 1 all have one valence electron, they must have similar chemical properties and bond in a similar way. This is true about every group on the periodic table. Group 15, for example, must bond in a similar way and have similar properties. 6. After completing the activity and watching the video, why do elements in the same group have similar properties? (4 points) Elements in the same group have similar properties and reactions because they have the same number of valence electrons.







