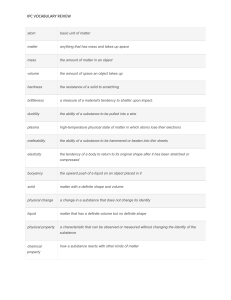
Chemistry Name________________________________ 1. An
advertisement

Chemistry Name________________________________ 1. An atom of which element has the greatest attraction for the electrons in a bond with a hydrogen atom? (1) chlorine (2) silicon (3) phosphorus (4) sulfur 2. Which property could be used to identify a compound in the laboratory? (1) mass (2) temperature (3) melting point (4) volume 3. Which statement describes what occurs as two atoms of bromine combine to become a molecule of bromine? (1) Energy is absorbed as a bond is formed. (3) Energy is absorbed as a bond is broken. (2) Energy is released as a bond is formed. (4) Energy is released as a bond is broken. 4. Given a formula for oxygen: O = O, What is the total number of electrons shared between the atoms represented in this formula? (1) 1 (2) 2 (3) 8 (4) 4 5. Which formulas represent two polar molecules? (1) CO2 and HCl (2) H2O and HCl (3) CO2 and CH4 (4) H2O and CH4 6. What is the total number of valence electrons in a sulfide ion in the ground state? (1) 8 (2) 16 (3) 2 (4) 18 7. As a bond between a hydrogen atom and a sulfur atom is formed, electrons are (1) shared to form an ionic bond (3) shared to form a covalent bond (2) transferred to form an ionic bond (4) transferred to form a covalent bond 8. Which element has an atom with the greatest attraction for electrons in a chemical bond? (1) As (2) N (3) Bi (4) P 9. Which formula represents a polar molecule? (1) Br2 (2) CH4 (3) CO2 (4) NH3 10. Magnesium and calcium have similar chemical properties because an atom of each element has the same total number of (1) electron shells (2) neutrons (3) valence electrons (4) protons 11. A barium atom attains a stable electron configuration when it bonds with (1) one chlorine atom (2) two chlorine atoms (3) one sodium atom (4) two sodium atoms 12. Which term indicates how strongly an atom attracts the electrons in a chemical bond? (1) alkalinity (2) atomic mass (3) electronegativity (4) activation energy 13. A solid substance is an excellent conductor of electricity. The chemical bonds in this substance are most likely (1) ionic, because the valence electrons are shared between atoms (2) ionic, because the valence electrons are mobile (3) metallic, because the valence electrons are stationary (4) metallic, because the valence electrons are mobile 14. When sodium and fluorine combine to produce the compound NaF, the ions formed have the same electron configuration as atoms of (1) argon, only (2) neon, only (3) both argon and neon (4) neither argon nor neon 15. Draw the Lewis Structure for CF4. Is this compound polar or nonpolar? Are the C – F bonds polar or nonpolar? 16. Atoms of which element have the greatest tendency to gain electrons? (1) bromine (2) fluorine (3) chlorine (4) iodine 17. Which element is a solid at STP and a good conductor of electricity? (1) iodine (2) nickel (3) mercury (4) sulfur 18. Given the balanced equation representing a reaction: Cl2(g) Cl(g) + Cl(g). What occurs during this change? (1) Energy is absorbed and a bond is broken. (3) Energy is absorbed and a bond is formed. (2) Energy is released and a bond is broken. (4) Energy is released and a bond is formed. 19. What is the net charge on an ion that has 9 protons, 11 neutrons, and 10 electrons? (1) 1+ (2) 1– (3) 2+ (4) 2– 20. Which two particles make up most of the mass of a hydrogen-2 atom? (1) electron and neutron (2) electron and proton (3) proton and neutron (4) proton and positron 21. Elements with atomic numbers 112 and 114 have been produced and their IUPAC names are pending approval. However, an element that would be put between these two elements on the Periodic Table has not yet been produced. If produced, this element will be identified by the symbol Uut until an IUPAC name is approved. (a) Draw a Lewis electron-dot diagram for an atom of Uut. (b) Determine the charge of a Uut nucleus. Your response must include both the numerical value and the sign of the charge. (c) Identify one element that would be chemically similar to Uut. 22. Which two characteristics are associated with metals? (1) low first ionization energy and low electronegativity (3) low first ionization energy and high electronegativity (2) high first ionization energy and low electronegativity (4) high first ionization energy and high electronegativity 23. An oxygen molecule contains a double bond because the two atoms of oxygen share a total of (1) 1 electron (2) 3 electrons (3) 2 electrons (4) 4 electrons 24. At STP, which element is brittle and not a conductor of electricity? (1) S (2) Na (3) K (4) Ar 25. What is the total number of electrons in a Mg2+ ion? (1) 10 (2) 14 (3) 12 (4) 24 26. At STP, fluorine is a gas and bromine is a liquid because, compared to fluorine, bromine has (1) stronger covalent bonds (3) stronger intermolecular forces (2) weaker covalent bonds (4) weaker intermolecular forces 27. Write the Lewis Structure for an atom of an element containing 5 protons, 5 electrons and 5 neutrons. 28. Is SF6 polar or nonpolar? Explain your answer in terms of electron distribution in the molecule. 29. A compound has a low melting point and is nonconductive as a liquid. The compound could be (1) Na (2) KCl (3) C2H6 (4) CuBr2 30. NH3 has a higher boiling point than CO2. Explain this observation in terms of intermolecular forces.