Calcium Hypochlorite SOP
advertisement

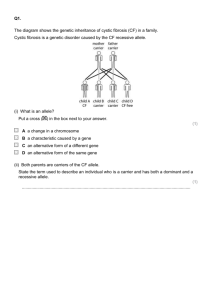
Standard Operating Procedure Calcium Hypochlorite Print a copy and insert into your Laboratory Safety Manual and Chemical Hygiene Plan. Refer to instructions for assistance. Department: Chemistry Date SOP was written: 11/28/2012 Date SOP was approved by PI/lab supervisor: Principal Investigator: Richmond Sarpong Internal Lab Safety Coordinator/Lab Manager: Lab Phone: 1/13/2013 Rebecca Murphy 510-643-2485 Office Phone: 510-643-6312 Emergency Contact: Richmond Sarpong, 626-644-2407 Location(s) covered by this SOP: Latimer 834, 836, 837, 838, 839, 842, 844, 847, 849, 907 (Name and Phone Number) (Building/Room Number) Type of SOP: ☐ Process ☒Hazardous Chemical ☐ Hazardous Class Purpose Calcium hypochlorite is an oxidizer that may cause a fire upon contact with combustibles or organic material. Contact with acids liberates toxic gas. It is harmful if ingested or absorbed through the skin and may be harmful if inhaled. It causes burns by all exposure routes. Calcium hypochlorite is mainly used as a disinfectant for drinking water and swimming pools and as a bleaching agent in household cleaners and laundry detergent. It is highly effective against algae, bacteria, slime, and fungi. Physical & Chemical Properties/Definition of Chemical Group CAS#: 7778-54-3 Class: Oxidizer, corrosive, harmful Molecular Formula: Ca(ClO)2 Form (physical state): Solid Color: Beige Calcium hypochlorite UCLA- EH&S 1 Date: 1/11/2013 CC/Reviewed by Boiling point: N/A Melting point: 100oC (212oF) Potential Hazards/Toxicity Calcium hypochlorite is an oxidizer that may intensify fires. Contact with combustibles and organic material may cause a fire. Contact with acids liberates toxic gas. It is harmful if ingested or absorbed through the skin and may be harmful if ingested. Material is extremely destructive to the tissue of the mucous membranes and upper respiratory tract. Causes severe skin burns and eye damage. Symptoms of exposure include inflammation and edema of the larynx and bronchi, pneumonitis, pulmonary edema, burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, and nausea. Engineering Controls NOTE: Specific information on engineering controls is to be added to the Protocol/Procedure section. Work with calcium hypochlorite should be conducted in a fume hood unless other controls are designated in the Protocol/Procedure section. Sash height should be kept low to avoid escaping fumes and provide a physical barrier. Consider wearing a face shield when handling the pure material. Personal Protective Equipment (PPE) Respirator Protection NOTE: Lab personnel intending to use/wear a respirator mask must be trained and fit-tested by EH&S. This is a regulatory requirement. Respirators should be used only under any of the following circumstances: As a last line of defense (i.e., after engineering and administrative controls have been exhausted). When Permissible Exposure Limit (PEL) has exceeded or when there is a possibility that PEL will be exceeded. Regulations require the use of a respirator. An employer requires the use of a respirator. There is potential for harmful exposure due to an atmospheric contaminant (in the absence of PEL) As PPE in the event of a chemical spill clean-up process Hand Protection Handle with gloves. Nitrile gloves (0.11mm) are recommended. Consideration should be given to double gloving given the possibility of extended contact. NOTE: Lab-specific and chemical-specific information on glove selection may be included in the Protocol/Procedure section. Refer to glove selection from the link below: For glove selection, usage.html. go to: http://ehs.berkeley.edu/hs/63-laboratory-safety/94-glove-selection-and- Eye Protection Safety glasses with side shields or tightly fitting safety goggles. Use face shield (8-inch minimum) when appropriate. Use equipment for eye protection tested and approved under appropriate government standards such as ANSI Z87.1, NIOSH (US) or EN 166(EU). Calcium hypochlorite UCLA- EH&S 2 Date: 1/11/2013 CC/Reviewed by Skin and Body Protection Long pants, closed-toed and closed-heeled shoes, cotton-based clothing/attire, and apron/lab coat of chemically resistant material must be worn for protecting against chemical hazards. Hygiene Measures Avoid contact with skin, eyes, and clothing. Wash hands before breaks and after handling. First Aid Procedures If inhaled Move person into fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Consult a physician immediately. In case of skin contact Take off contaminated clothing immediately. Wash off with soap and plenty of water for 15 minutes. Take victim immediately to hospital. Consult a physician. In case of eye contact Flush eyes with plenty of water for at least 15 minutes using an emergency eyewash station, lifting upper and lower eyelids and removing contact lenses. Immediately consult a physician. Continue rinsing eyes during transport to hospital. If swallowed Do not induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician. Special Handling and Storage Requirements Working alone Certain extremely hazardous operations should not be performed if the PI or Lab Safety Contact(s) are not present. Never work alone with extremely hazardous materials/operations. See the Protocol/Procedure section below for specific prohibitions (if any) on working alone. Precautions for safe handling: Avoid contact with skin, eyes, and clothing. Avoid inhalation and ingestion. Provide adequate ventilation. Keep away from heat and sources of ignition- No smoking. Conditions for safe storage: Keep container tightly closed in a cool, dry, and well-ventilated area. Never allow contact with water during storage. Incompatible with organic materials, acids, amines, ammonia, alcohols, reducing agents, and metals. Do not store near acids. Spill and Accident Procedure Chemical Spill Dial 911 and 510-642-9090 Spill – Assess the extent of danger. Help contaminated or injured persons. Evacuate the spill area. Avoid breathing vapors. If possible, confine the spill to a small area using a spill kit or absorbent material. Keep others from entering contaminated area (e.g., use caution tape, barriers, etc.). Small (<1 L) – If you have training, you may assist in the clean-up effort. Use appropriate personal protective equipment and clean-up material for chemical spilled. Double bag spill waste in clear plastic bags, label and take to the next chemical waste pick-up. Large (>1 L) – Dial 911 and EH&S at 510-642-9090 for assistance. Calcium hypochlorite UCLA- EH&S 3 Date: 1/11/2013 CC/Reviewed by Chemical Spill on Body or Clothes – Remove clothing and rinse body thoroughly in emergency shower for at least 15 minutes. Seek medical attention. Notify supervisor and EH&S at 510-642-9090 immediately. Chemical Splash Into Eyes – Immediately rinse eyeball and inner surface of eyelid with water from the emergency eyewash station for 15 minutes by forcibly holding the eye open. Seek medical attention. Notify supervisor and EH&S at 510-642-9090 immediately. Medical Emergency Dial 911 or 510-642-9090 Life Threatening Emergency, After Hours, Weekends And Holidays– Dial 911 or go to the nearest emergency room. Note: All serious injuries must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Non-Life Threatening Emergency – Go to the Occupational Health Facility (Tang Health Center). After hours go to the nearest emergency room. Note: All serious injuries must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Needle stick/puncture exposure (as applicable to chemical handling procedure) – Wash the affected area with antiseptic soap and warm water for 15 minutes. For mucous membrane exposure, flush the affected area for 15 minutes using an eyewash station. Go to the Occupational Health Facility (Tang Health Center). After hours go to the nearest emergency room. Note: All needle stick/puncture exposures must be reported to EH&S within 8 hours. Follow up with a call to 510-642-9090 to report the incident. Decontamination/Waste Disposal Procedure Wearing proper PPE, decontaminate equipment and bench tops with soap and water. Sweep up or shovel spills avoiding dust formation. Dispose of the used chemical and contaminated disposables as hazardous waste following the guidelines below. General hazardous waste disposal guidelines: Label Waste Preparation Reactivity/ Stability: Calcium hypochlorite is a powerful oxidizing agent, particularly in the presence of water or at higher temperature as Calcium hypochlorite decomposes to release oxygen and chlorine gases. Calcium hypochlorite is noncombustible, but Calcium hypochlorite will accelerate the burning of combustible materials. Conditions to Avoid: Avoid heat, flames, sparks and other sources of ignition Incompatibilities/Materials to Avoid: water, reducing agents, combustible material, phenol When not in use, keep container tightly closed in a dry and well-ventilated place. Store and transport THF containers in secondary containment (for example polyethylene bottle carrier). Know the location of the nearest fire extinguisher, eyewash, and safety shower before beginning work. Calcium hypochlorite UCLA- EH&S 4 Date: 1/11/2013 CC/Reviewed by Eliminate incompatible materials from the potential spill area. Lab-specific Information All work for this procedure is to take place in the designated fume hood. Wear proper PPE. Keep calcium hypochlorite containers tightly closed in a dry and well-ventilated place away from incompatible substances. • Label all containers with the label provided at http://ehs.berkeley.edu/hm/279-new-hazardouswaste-program-hwp.html. See the EH&S Fact Sheet, “Hazardous Waste Management” for general instructions on procedures for disposing of hazardous waste. Dispose of Waste • Dispose of regularly generated chemical waste within 6 months. • Call EH&S for questions. Safety Data Sheet (SDS) Location Online SDS can be accessed at SDS can be accessed online at http://ucmsds.com Protocol/Procedure for Calcium Hypochlorite Procedure/ Use calcium hypochlorite is used in the lab as a bleaching agent. Scale 2 dry oz. (56 grams) in 1 gallon of water = 1% solution or 10,000 ppm. Calcium hypochlorite UCLA- EH&S Engineering Controls/Equipment All work using calcium hypochlorite must be performed in a ventilated fume hood. PPE (eye, face, gloves, clothing) Eye Protection: Wear tight-fitting safety goggles or safety glasses with side shields. Eliminate ignition sources such as open flames, hot surfaces, steam baths, static electricity, and operation of mechanical and electrical equipment that is not intrinsically safe. Face protection: Wear a face shield Gloves: Wear disposable, nitrile gloves (0.11mm). Clothing: Wear apron/lab coat (100% cotton based); cotton based clothing/attire; fulllength pants or equivalent; and close-toed, closedheeled shoes. 5 Procedure Steps and Precautions Because calcium hypochlorite is a strong oxidant, it may produce chemical burns. Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handing calcium hypochlorite Avoid breathing vapors, mist or gas. Use only a clean, dry scoop (dedicated for this use) made of metal or plastic each time calcium hypochlorite is taken from the container. Add calcium hypochlorite only to water. A fire or explosion may result if calcium hypochlorite is mixed with other Date: 1/11/2013 CC/Reviewed by materials contaminated with acids or brought into contact with any easily combustible materials such as oil, kerosene, gasoline, paint products and any other organic materials. NOTE Any deviation from this SOP requires approval from PI. Documentation of Training (signature of all users is required) Prior to conducting any work with calcium hypochlorite, designated personnel must provide training to his/her laboratory personnel specific to the hazards involved in working with this substance, work area decontamination, and emergency procedures. The Principal Investigator must provide his/her laboratory personnel with a copy of this SOP and a copy of the SDS provided by the manufacturer. The Principal Investigator must ensure that his/her laboratory personnel have attended appropriate laboratory safety training or refresher training within the last one year. I have read and understand the content of this SOP: Name Signature Initials Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. UCLA- EH&S Date Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter text. Calcium hypochlorite Identification 6 Date: 1/11/2013 CC/Reviewed by Click here to enter text. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter a date. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. Calcium hypochlorite UCLA- EH&S 7 Date: 1/11/2013 CC/Reviewed by Click here to enter text. Click here to enter a date. Click here to enter a date. Click here to enter text. Calcium hypochlorite UCLA- EH&S 8 Date: 1/11/2013 CC/Reviewed by