L13 - Handout (States of Matter)
advertisement

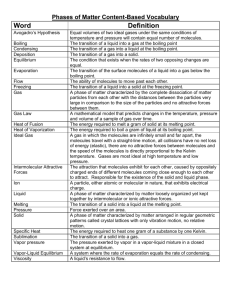
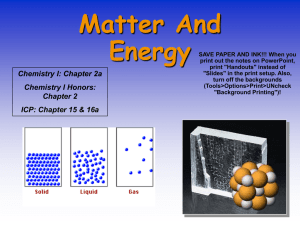
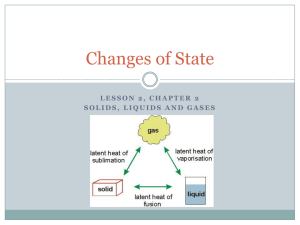
States of Matter Solid, Liquid or Gas?? The state of a pure substance (solid, liquid or gas) depends on the strength of the attractive forces between its particles. In Solid State: The attractive forces are relatively strong and each particle remains bonded to its adjacent particles. As the solid is heated up, the average kinetic energy (temperature) of its particles increases. Eventually, the particles have enough kinetic energy (motion) to break the bonds with neighbouring particles. At this particular temperature (its melting point), the particles transition into liquid form. At the melting point, all added energy is used to disrupt the intermolecular forces between the solid particles. This involves a change in potential energy. During this phase change (known as fusion), there will be no change in temperature until the entire sample has melted. In Liquid State: The particles can move past each other but they are constantly breaking and forming bonds with neighbouring particles. As the liquid is heated up, their kinetic energy increases until the particles have enough energy to break all the intermolecular bonds and remain free of other particles. At this particular temperature (its boiling point), the particles transition into a gas. At the boiling point, all added energy is used to disrupt all the intermolecular forces between the liquid particles. This involves a change in potential energy. During this phase change (known as vaporization), there will be no change in temperature until the entire sample has transformed into a gas. The particles in a solid are rigidly attached to one another and often form a symmetrical structure. In a liquid, the particles are always in contact but can readily move past one another. In a gas, the only contacts between particles are collisions. The particles remain apart from one another after the collisions. Melting and Boiling Points When metals melt or boil, metallic bonds must be broken. Metals When ionic compounds melt or boil, ionic bonds must be broken. Ionic Compounds When molecular compounds melt or boil, intermolecular forces must be overcome. The covalent bonds between atoms within a molecule don’t break when the compound melts or boils. Molecular Compounds Substance Melting Point (oC) Boiling Point (oC) Substance Melting Point (oC) Boiling Point (oC) Substance Melting Point (oC) Boiling Point (oC) Li(s) +181 +1342 CsBr(s) +636 +1300 H2(g) -259 -253 Sn(s) +232 +2602 NaI(s) +661 +1304 Cl2(g) -102 -34 Al(s) +660 +2519 MgCl2(s) +714 +1412 H2O(l) 0 +100 Ag(s) +962 +2162 NaCl(s) +801 +1465 C6H6O(l) +41 +182 Cu(s) +1085 +2562 MgO(s) +2825 +3600 C6H12O6(s) +146 decomposes The melting points for metals and ionic compounds are much higher than those of molecular compounds. The same trend is observed for the boiling points. Metallic bonds and ionic bonds are formed by the electrostatic attractive forces between whole positive and negative charges, which create very strong bonds. Within the ionic compounds, note that the magnesium oxide (MgO) has much higher melting and boiling points. Magnesium (2+) and oxide (2-) have larger charges than most of the ions in the list, which have a 1+ or 1charge. Larger charges create a much stronger attractive force between ions. The intermolecular forces between molecules result from the interaction between partial positive and negative charges, which are much weaker than ionic and metallic bonds. Much less energy is required to break the weaker bonds between molecules in molecular compounds. Consequently, they have much lower melting and boiling points. Although molecular compounds have much lower melting and boiling points, there is large variability of melting and boiling points among the molecular compounds. The Melting and Boiling Points of Diatomic Molecules Substance Number of electrons in a molecule Melting Point (oC) Boiling Point (oC) H2(g) 2 -259 -253 N2(g) 14 -210 -196 O2(g) 16 -219 -183 *Note: The substances that have similar numbers of electrons also have similar melting and boiling points. *Note: As the number of electrons in these molecules increases, Cl2(g) 34 -102 -34 the melting and boiling points also increase as Br2(l) 70 -7.2 +59 a result of the stronger London (dispersion) I2(s) 106 +114 +184 forces. All of these molecules are symmetrical, non-polar molecules with a linear shape. Therefore, the only interactions between the molecules are LDFs (London forces) F2(g) 18 -220 -188 The Melting and Boiling Points of Molecules of Similar Size Substance Number of electrons in a molecule Melting Point (oC) Boiling Point (oC) H2O(l) 10 0 +100 H2S(g) 18 -86 -60 CO2(g) 22 (sublimation point) -78 In these compounds that have similar numbers of electrons, the previous trend is not clearly observed. Since carbon dioxide becomes a gas at temperatures lower than the boiling points of the other two, it must have the weakest intermolecular interactions. This can be explained as it is the only non-polar molecule (it has a linear shape AX2) in the list. Therefore, although it may have the strongest of the London (dispersion) forces, it does not also have the dipole-dipole interactions found in the other two molecules. Water and hydrogen sulphide are both bent molecules (AX2E2) and are polar. Yet why does water (with fewer electrons and therefore weaker London forces) actually have much higher melting and boiling points? Water molecules have stronger attractive forces between them because of the hydrogen bonding that takes place between water molecules. Hydrogen bonding is not evident between hydrogen sulphide molecules. The following graph will help to illustrate the influence of hydrogen bonding. (The dotted line shows what the boiling point of water would probably be if it had no hydrogen bonds.) The hydrides of the Group 14 elements are all symmetrical molecules with identical bonded atoms. They are all tetrahedral (AX4) and non-polar. As a result, the only intermolecular forces present are London (dispersion) forces and the trend of increasingly larger molecules having increasing higher boiling points is observed with no exception. The hydrides of the Group 15, 16, and 17 elements are all symmetrical molecules but they are all polar. Group 15 hydrides (AX3E) are all trigonal pyramidal, Group 16 hydrides (AX2E2) are all bent, and the Group 17 hydrides (AXE3) are all linear. Furthermore, in the molecules of these hydrides in which the central atom is from period 2 (N, O and F), the dipole-dipole interaction is particularly strong (hydrogen bonding). Chem 20 States of Matter Assignment 1. Predict which substances will have the higher boiling point in each of the following pairs. Explain your predictions using bonding theories. a. NH2Cl or PH2F c. NH4Cl or CH3Br e. CO2 or SO2 b. Ne or Xe d. CH4 or C2H6 f. CH3OH or C2H5NH2 2. Compare the bonding and molecular polarity of SeO3(s) and SeO2(s). 3. List the following substances in order of increasing boiling points: C3H8, ZnO, C7H15OH, and C5H12. Give a reason for your answer. 4. The alkanes are a family of non-polar molecular compounds containing carbon and hydrogen. The data below contains the melting points and boiling points for alkanes having 1 to 10 carbon atoms. Using graph paper (and a ruler!!|!), draw a graph representing this data. Your graph should be a labelled line graph that plots temperature (on the y-axis) vs. molecular electron count (on the x-axis). Name Formula methane Number of Electrons per molecule Melting Point (oC) Boiling Point (oC) CH4 -182 -161 ethane C2H6 -183 -89 propane C3H8 -188 -42 butane C4H10 -138 -1 pentane C5H12 -130 +36 hexane C6H14 -95 +69 heptane C7H16 -91 +98 octane C8H18 -57 +126 nonane C9H20 -53 +151 decane C10H22 -30 +174