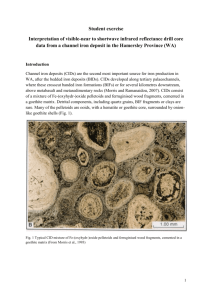
hematite and goethite inclusions in low grade dolomitic banded iron
advertisement

HEMATITE AND GOETHITE INCLUSIONS IN LOW GRADE DOLOMITIC BANDED IRON FORMATIONS: MAGNETISATION DURING HEATING EXPERIMENTS AND ITS IMPLICATIONS FOR ORE BENEFICIATION Beate Orberger1, Alina Tydryn1, Christiane Wagner2, Benoît Baptiste3, Richard Wirth4, Rachael Morgan1, Serge Miska1 1 Université Paris-Sud, Laboratoire GEOPS, UMR 8148 (CNRS-UPS), Bât. 504, 91405 Orsay, France; beate.orberger1@orange.fr 2 Sorbonne Universités, UPMC, Univ Paris 06, CNRS, ISTeP, 4 Place Jussieu, F-75005, Paris, France. 3 Sorbonne Universités, UPMC, Univ Paris 06, CNRS, IMPMC, 4 Place Jussieu, F-75005, Paris, France. 4 Helmholtz Centre Potsdam, GFZ German Research Centre for Geosciences, Section 3.3, Telegrafenberg, 14473-Potsdam, Germany Banded iron formations comprise complex textures and mineralogies, which result from fluid-rock interaction related to high and low temperature alteration. The initial ironhydroxyde mineralogy and associated phases such as carbonates, quartz, apatite and phyllosilicates were transformed leading to an upgrading of these BIF’s into the world’s largest source of iron ore. In low grade BIFs, a large part of the Fe is related to nanometric iron bearing inclusions within micrometric quartz and/or carbonates (mainly dolomite). We studied laminated BIF samples from a drill core containing 27 wt.% Fe2O3, 0.2 wt.% SiO2, 0.32 wt.% MnO, 15.46 wt.% MgO, 22.32 wt.% CaO, 0.09 wt. % P2O5, 0.15 wt. % H2O and 34.08 wt. % CO2 (Àguas Claras Mine, Quadrilátero Ferrífero, Brazil). Bright rose coloured dolomite and quartz bands alternate with massive specular hematite bands. Ramanspectroscopy, X-ray diffraction and FIB-TEM analyses reveal that the nanoinclusions in dolomite are mainly hematite and minor goethite, partly occurring as clusters, which are derived from fluid inclusions (Fig. 1). Curie Balance analyses were carried out at different heating steps and temperatures on whole rock samples and a synthetic mix of decarbonated sample and pure dolomite. X-ray diffraction (Co- and Cu-tube) show that new phases: lime (CaO), periclase (MgO), portlandite (Ca(OH) 2), magnesioferrite (MgFe204) and srebrodoskite (Ca2Fe2O5) were formed when samples heated until 920°C. These new sample parametres: newly formed strongly magnetic magnesioferrite accompanied by a textural modification can be used for an optimized metallurgical process design. Keywords: iron ore, banded iron formations, hematite, magnesioferrite, srebrodoskite, magnetic behavior, ironoxide inclusions, carbonates Figure 1: TEM images of the dolomite band A) HAADF overview of the dolomite foil showing low angle grain boundary between two dolomite crystals (seen in middle) with inclusions of euhedral hematite (he) and clusters of nanometric goethite and ferrihydrite in the porous dolomite; B) EDX analysis on the HAADF image of the dolomite composition. The Cu signal is from the copper grid whilst the Ga signal results from the FIB-TEM preparation; C. Bright field image of the euhedral hematite showing its heterogeneous contrast due to high dislocation density; D. Bright field image of idiomorphic goethite in a cluster. Circular decomposition structures caused by the electron beam are visible in the lath (inset); E and F. Clusters of iron hydroxides as a pore filling, most likely derived from a fluid inclusion; G. A Fast Fourier Transform (FFT) diffraction pattern of D. From the pattern it is not clear to identify goethite or ferrihydrite, however, idiomorphic shapes indicate goethite rather ferrihydrite (the latter is poorly crystalline); H. EDS spectra of goethite indicating the presence of Si, which retards its transformation into hematite.