Project 2 - Chemistry Courses: About
Multistep Synthesis
Pre-lab questions:
1.
Draw a structure of the final product and indicate which reagent contributed each of the carbons in the final product.
2.
When a crossed aldol reaction is performed with a ketone as one partner, how is one major product produced? How does the procedure play an important role?
3.
Draw a mechanism for each step of this reaction sequence. What is an aldol reaction, whaat is a Dieckmann annulation? What is the difference between hydrolysis, decarboxylation, and dehydration?
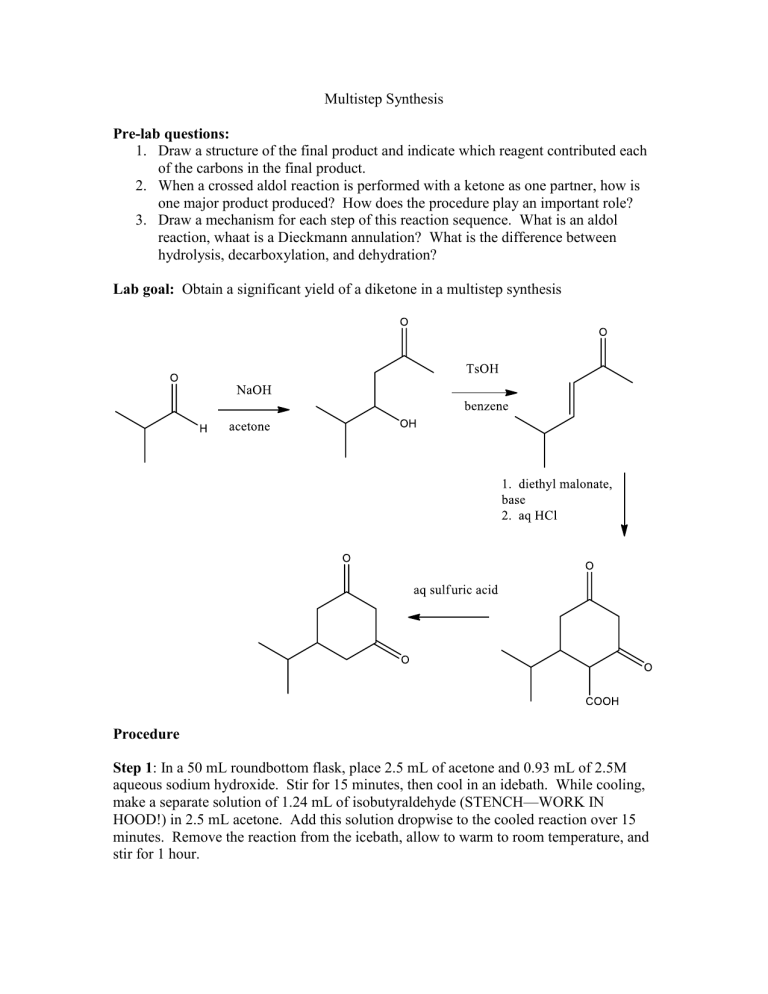
Lab goal: Obtain a significant yield of a diketone in a multistep synthesis
Procedure
Step 1 : In a 50 mL roundbottom flask, place 2.5 mL of acetone and 0.93 mL of 2.5M aqueous sodium hydroxide. Stir for 15 minutes, then cool in an idebath. While cooling, make a separate solution of 1.24 mL of isobutyraldehyde (STENCH—WORK IN
HOOD!) in 2.5 mL acetone. Add this solution dropwise to the cooled reaction over 15 minutes. Remove the reaction from the icebath, allow to warm to room temperature, and stir for 1 hour.
Neutralize the reaction with 10% HCl dropwise. Once the reaction is pH 7 by pH paper, dilute it with 10 mL of water. Extract the reaction mixture with ether (3 x 8 mL)Combine the organic layers and wash them with 10 mL brine. Transfer the organic layer to an
Erlenmeyer flask and dry with 3 g of anhydrouse sodium sulfate. Filter the solution with a Buchner funnel with filter paper, wash the solids with a little ether. Transfer the ether solution to a tared roundbottom flask and evaporate. (Do not heat the solution because the product is somewhat volatile. After the rotovap is done, you should have about 1.5 g of oily material. Blow a little air or nitrogen over the oil to remove trace ether.
Data: IR and proton NMR (save proton NMR for next week)
Step 2 : Prior to the reaction, get together with 3 partners and make an NMR sample of week one. Each partner should contribute one drop of product 1 to mix in CDCl
3
for the
NMR sample.
In a 25 mL rounbottom flask, add 0.25 g TsOH monohydrate and 4 g anhydrouse sodium sulfate. Use benzene to transfer your product from step one to the reaction. (You should add 5 mL of anhydrouse benzene total.) Attach a reflux condenser and heat for 2.25 hours. Monitor the level of benzene. If it is low, you may add a 2 mL portion.
TLC: Obtain a TLC of the reaction at the beginning, at the half-way mark, and at the end of the reaction. Run the TLC in 1:4 ethyl acetate: hexanes. Look at the plate by UV light, then stain with anisaldehyde.
During this time, you may also obtain the proton NMR of the step one product.
Once the reaction is done, cool it to room tempearature an dneutralize it with saturated sodium bicarb solution until it is pH 7 by pH paper. Dilute the solution with 10 mL of water, and extract the reaction with ether (3 x 10mL). Combine the organic layers and wash with 10 mL of brine. Transfer the organic layer to an Erlenmeyer flask and dry with 3 g of anhydrouse sodium sulfate. Filter the solution with a Buchner funnel with filter paper, wash the solids with a little ether. Transfer the ether solution to a tared roundbottom flask and evaporate. (Do not heat the solution because the product is somewhat volatile. After the rotovap is done, you should have about 1.0 g of oily material. Blow a little air or nitrogen over the oil to remove trace ether.
Data: TLC and proton NMR (next week)
Step 3: Prior to the reaction, get together with 3 partners and make an NMR sample of week one. Each partner should contribute one drop of product 2 to mix in CDCl
3
for the
NMR sample.
In a 100 mL roundbottom flask add 15 mL of absolute ethanol. Add 3.5 mL of sodium ethoxide (21% solution) dropwise with a CLEAN, DRY needle and syringe. (Caution— reacts with water. See AI for assistance.) Add 1.4 mL of diethyl malonate dropwise.
Attach a reflux condenser, heat the reaction to reflux, and allow to reflux for 15 minutes.
During this time, dissolve your product from step 2 in 5 mL of absolute ethanol. After the 15 minute reflux is complete, add your reaction solution dropwise through the condenser. Stir at reflux for 30 more minutes. Add 5.1 mL of 3.8 M potassium hydroxide through the condenser, and reflux for 2 hours. Cool the solution to room temperature, remove the condenser, and neutralize the reaction with 10% HCL dropwise to pH 7 according to pH paper. (Store this solution for next time.)
Step 4: Spot a TLC plate with your reaction mixture from step 3, and set the plate aside.
Using a rotovap, remove the ethanol from the step 3 reaction (water will remain with your crude reaction mixture.) Connect a reflux condenser to the flask and heat to a gentle reflux. Add 6 mL of a 1:5 sulfuric acid: water solution through the condenser dropwise.
Continue heating until no more carbon dioxide bubbles form. (This might take about 1 hour.) Cool the reaction to room temperature, and add saturated sodium bicarb solution until the pH is 4 by pH paper.
Extract the reaction mixture with methylene chloride (3 x 10 mL.) Combine the organic layers and wash with 10 mL of brine. Transfer the organic layer to an Erlenmeyer flask and dry with 3 g of anhydrouse sodium sulfate. Filter the solution with a Buchner funnel with filter paper, wash the solids with a little methylene chloride. Transfer the methylene chloride solution to a tared roundbottom flask. Spot your TLC plate from the beginning of the reaction with your final product solution, and develop it in a 4:1 ethyl acetate:hexanes solvent. Evaporate the methylene chloride to give your final product as a brown oil. Obtain an IR of the material.
Observations and Results
You know how to do it by now! Keep good records!
Discussion:
1.
Step 1—How does IR support the identity of the product? Based on NMR, how pure is your sample? Predict the splitting and chemical shift you expect to see for each proton in your step 1 compound. How would you describe the multiplicity of each peak? Compare your predictions to your NMR data. Does it match what is expected? Draw a mechanism for the reaction. What is an expected side product? What is the % yield for this first step?
2.
Step 2—Provide a mechanism for this reaction. How is the TLC data useful for determining if the reaction went to completion? What are the R f
values for the first intermediate and the second intermediate? Are their relative values what you would expect based on the polarity of each molecule? Does the proton NMR support the identity of the product? How can you use this data to determine if the double bond is cis or trans? In many cases, you can observe a mix of cis and trans product—does your NMR spectrum support
this observation? Can you determine a relative product distribution between cis and trans alkene product? What is the % yield for the second step?
3.
Steps 3-4—Is TLC data consistent with your expectations for steps 3 and 4?
How pure is your final product? What data support the identity of the product? Is the IR consistent with a decarboxylation reaction? Draw a mechanism for the annulation step of the reaction. Why are a hydrolysis and decarboxylation necessary to get to the final product? What is the overall % yield for the reaction? (You don’t need to determine the yields fo steps 3 and
4.)
Conclusion: Summarize the main conclusions of the lab
Reference(s) Did you use any authentic data?











