CHH Review Unit 2
advertisement

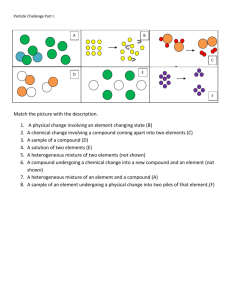
Name:____________________________ Date:___________ Period:____ Review Chemistry I Honors Unit 2: Properties of Matter 1. The two general properties that all matter shares are _____________ and _______________. 2. Place a check mark next to each item that is matter which is made of atoms (has mass and takes up space). ____a. apple ____b. air ____c. light ____d. water ____e. RNA ____f. heat ____g. proton ____h. oil ____i. ice ____j. radio waves 3. List the 4 states of matter, and circle the correct descriptions of their properties. 1. _______________ 3. _______________ shape: indefinite or definite shape: indefinite or definite volume: indefinite or definite volume: indefinite or definite moving: a lot or a little or not moving: a lot or a little or not 2. _______________ 4. _______________ shape: indefinite or definite shape: indefinite or definite volume: indefinite or definite volume: indefinite or definite moving: a lot or a little or not moving: a lot or a little or not 4. The diagram below a simple water cycle. Label each physical phase change in the appropriate box. deposition 1 5. Classify each type of substance is represented in diagrams 1-4 as element, compound, or mixture. Diagram Key: atom A atom B (1) Classification: (2) ____________ ____________ (3) (4) ____________ ____________ 6. Which of the diagrams from question 5 represent pure substances? How could you tell? ____________________________________________________________ (make sure you understand this explanation and ask if you need more discussion about it) 7. Classify the following materials as: element compound homogeneous mixture Can be separated by _____ means. heterogeneous mixture (circle one) a. cheeseburger ___________________ physical chemical NO b. salt water ___________________ physical chemical NO c. air ___________________ physical chemical NO d. hydrogen gas (H2) ___________________ physical chemical NO e. silver (Ag) ___________________ physical chemical NO f. steel ___________________ physical chemical NO g. steam (H2O) ___________________ physical chemical NO h. ice cream sundae ___________________ physical chemical NO i. table salt (NaCl) ___________________ physical chemical NO j. carbon dioxide (CO2) ___________________ physical chemical NO k. ice (H2O) ___________________ physical chemical NO 8. For the materials listed in question 7, list all that are pure substances. (recall your #6 answer above) 2 9. Mixtures are materials that that can be separated by physical means. Describe one method to separate a heterogeneous mixture: Name and describe one method to separate a homogeneous mixture: 10. A student tries to identify three unknown white solids by observing and recording the data listed below (a-e). Which property would be the best to identify this unknown solid? A. white crystalline solid B. mass is 7.00 g C. volume is 2.0 mL D. density is 3.50 g/mL E. insoluble (does not dissolve) in water 11. A few properties of aluminum are listed. Classify each property as a chemical or physical property. _________________ a. very malleable and ductile _________________ b. reacts with HCl to produce H2 gas _________________ c. thin, white coat of aluminum oxide forms the surface _________________ d. melting point is 660.3 C _________________ e. density is 2.70 g/mL 12. Classify the following as either a physical change (P) or a chemical change (C). a. _____folding paper into origami bird b. _____roasting marshmallows c. _____ burning wood d._____ cutting hair e._____ dissolving sugar in water f. _____ digesting a cheeseburger g. _____ painting a wall 3 13. Two clear liquids are mixed together in a beaker. A student observes and records the following data. a. bubbles form and a gas is released b. the temperature of the beaker increases from 22.0 oC to 36.0 oC. c. a blue gel forms and settles to bottom of the beaker d. a clear liquid remains in the beaker Which of the observations listed above indicate that a chemical change has occurred? 14. What type of substance could be separated into different parts by filtration as pictured below? 15. Below is a Hoffman electrolysis apparatus. Explain for what this device is used. Is this a physical or chemical change? How do you know? 4 Match each item with the correct statement below. A. element D. physical change B. compound E. heterogeneous C. chemical change F. homogeneous ___ 16. describes mixture with a non-uniform composition (has different parts) ___ 17. substance that cannot be changed into simpler substances by physical means ___ 18. cannot be broken down by chemical means ___ 19. does not affect the composition of the matter 20. Which of the following is NOT a solution? A. air B. salt water C. stainless steel D. soil 21. Suspensions and ________________ are both heterogeneous mixtures with larger particles than ___________ , but only the very large particles in ____________________ settle out due to gravity. 22. The Tyndall effect can distinguish between a _________________ and a solution by the _________________ of light. 23. What is one difference between a mixture and a compound? A. B. C. D. A compound has the same properties as the elements that form it. A compound can only be separated physically. A mixture can be separated physically. A mixture must be uniform in composition. 24. Identify each physical property as characteristic of metals (M), nonmetals (NM), or metalloids (MD): a. ___________ malleable b. ___________ poor conductor of heat c. ___________ brittle d. ___________ ductile e. ___________ good conductor of electricity f. ___________ brittle and good conductor of electricity g. ___________ good conductor of heat 5 KEY 1. mass and volume 2. a, b, d, e, g, h, i 3. solids: definite volume, definite shape, particles vibrate in place (moving a little), not compressible liquids: definite volume, indefinite shape, particles are close together but can move past each other, (moving a little), not easily compressible gases: indefinite volume, indefinite shape, takes the shape of its container, particles are very far apart, (moving a lot), easily compressible plasma: indefinite volume, indefinite shape, occurs only at extremely high temperatures. Most electrons have been stripped away creating ionized matter. (moving a whole lot!) 4. Absorbs Energy (endothermic) : melting, vaporization, sublimation Releases Energy (exothermic) : freezing, condensation, deposition(frost formation) 5. 1. element 2. mixture 3. compound 4. element 6. diagrams 1,3,4 …because each particle is identical. 7. a. heterogeneous mixture b. homogeneous mixture c. homogeneous mixture d. element e. element f. homogeneous mixture g. compound h. heterogeneous mixture i. compound j. compound k. compound 8. d, e, g, i, j, k 9. homogeneous separation by: Distillation heterogeneous separation by: Filtration, Using a magnet, Sorting by hand, Sifting 10. density 11. chemical properties: b, c 12. a. P b. C physical properties: a, d, e c. C d. P e. P f. C g. P 13. chemical change: a, b, c 14. heterogeneous mixture 15. The Hoffman electrolysis apparatus uses electric energy to split (decompose) the water molecule into hydrogen gas and oxygen gas. The hydrogen gas collects at the negative electrode called the cathode and oxygen gas collects at the positive electrode called the anode. This is a chemical change. A compound is broken down into two elements (new substance). 16. E 17. B 18. A 19. D 21. colloids , solutions , suspensions 24. a. M b. NM c. NM 20. D 22. colloid , scattering d. M e. M f. MD 23. C g. M 6