Stinging Jellyfish, Sea Grass, and Algae oh my! By Van Wagner
advertisement

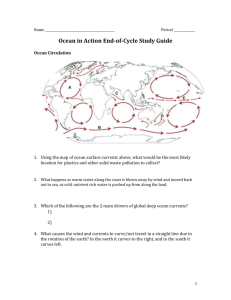
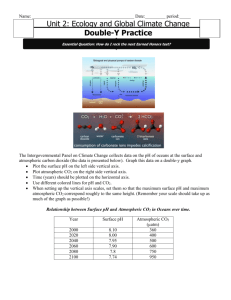
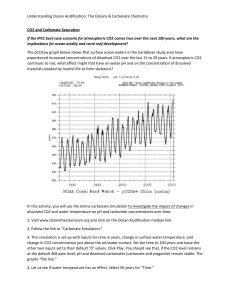
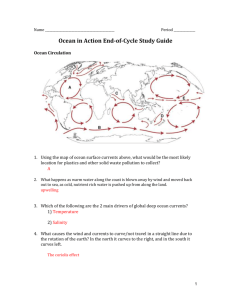
Stinging Jellyfish, Sea Grass, and Algae oh my! By Van Wagner April 2011 People are growing more and more aware of the climatic dangers of increased carbon dioxide, CO2, in our atmosphere especially in regards to how it is warming our planet. Fewer people realize that this CO2 is also changing the chemistry of our Oceans. When CO2 is mixed with water, H20, carbonic acid is produced, H2CO3. This mixing is natural and is caused by wind and wave power. What isn’t natural is the source of increased CO2; especially the burning of fossil fuels like coal, oil, and natural gas. Scientists know that the carbon going into the Ocean is from fossil fuels because it is different than the specific Carbon 14 isotope found in living organisms. Ancient carbon released from the burning of fossil fuels contains little to no Carbon 14. Currently, about 40% of our CO2 from fossil fuels remains in the atmosphere. The remaining 60% is deposited about evenly into vegetation and the ocean. In the Ocean, this CO2 is lowering the pH of the waters. This is especially the case on the surface (the top 50 meters). Ocean water is naturally a little alkaline with a pH about 8.2. Current data suggests a pH drop of 0.3 by 2100 is possible given our trends in fossil fuel use. While this number may seem small, the hazards to Ocean organisms is quite severe. A vast amount of sea-life requires Calcium Carbonate, CaCO3, for their exterior skeletons (oysters, star fish, and urchins for example). Even more organisms depend on CaCO3 for habitat. Coral reefs are exactly that; CaCO3. Coral reefs are created by organisms called Ocean Polyps. They are tiny animals that deposit thin layers of CaCO3 on top of previous layers. They are the building crews of coral reefs. As CO2 becomes higher, Ocean waters become more acidified making carbonate ions more scarce in the water. Coral Polyps expel more and more energy obtaining required carbonate until eventually they die. Considering the tremendous variety of organisms that require coral reefs for some portion of their life cycle, the cost of losing coral reefs would be devastating. Scientists have been studying areas of the sea that are already natural acidic due to CO2 released from underwater volcanic sources. Very few organisms can tolerate such acidified waters. These volcanic study areas have vast amounts of stinging jellyfish, sea grass, and algae (think of all of these as weedy species). The rich variety of organisms seen currently in healthy waters is not seen here. These areas serve as a sobering glimpse into the future because the environmental conditions present mimic what is expected to be the global norm by 2100 if we do not curb our use of fossil fuels. Sources for the data presented in my blog: Doney (2006) Scientific American “The Dangers of Ocean Acidification” Kolbert (April, 2011) National Geographic Magazine “The Acid Sea”