1990-Spring-Exam-1
advertisement
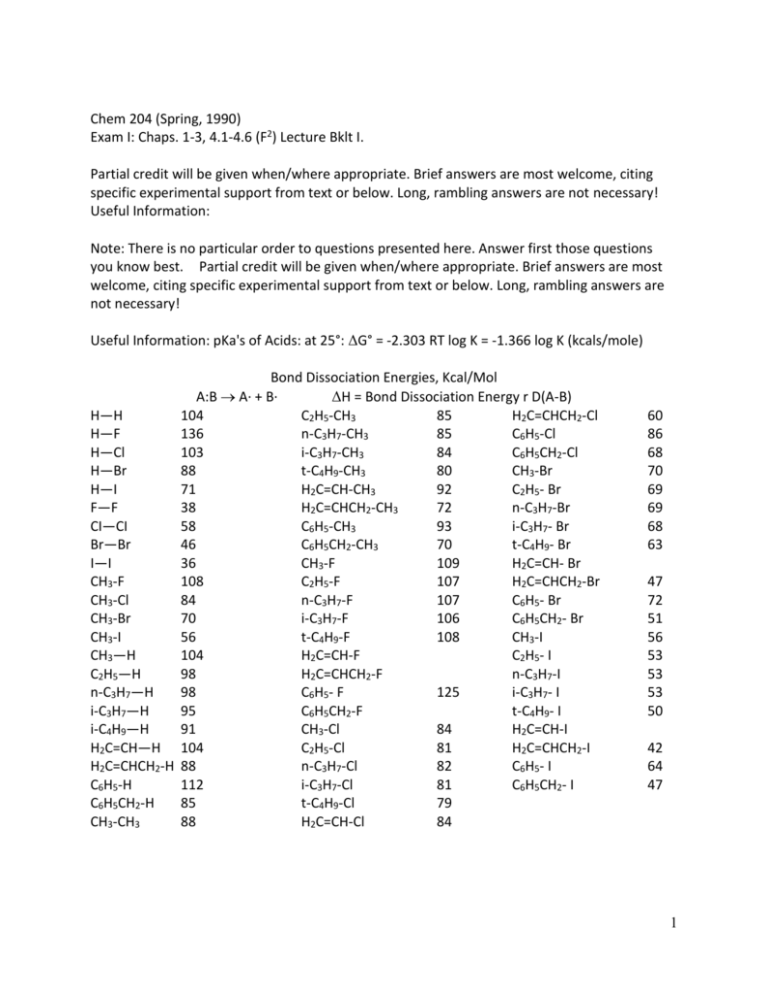
Chem 204 (Spring, 1990) Exam I: Chaps. 1-3, 4.1-4.6 (F2) Lecture Bklt I. Partial credit will be given when/where appropriate. Brief answers are most welcome, citing specific experimental support from text or below. Long, rambling answers are not necessary! Useful Information: Note: There is no particular order to questions presented here. Answer first those questions you know best. Partial credit will be given when/where appropriate. Brief answers are most welcome, citing specific experimental support from text or below. Long, rambling answers are not necessary! Useful Information: pKa's of Acids: at 25°: ΔG° = -2.303 RT log K = -1.366 log K (kcals/mole) Bond Dissociation Energies, Kcal/Mol A:B A∙ + B∙ ΔH = Bond Dissociation Energy r D(A-B) H—H 104 C2H5-CH3 85 H2C=CHCH2-Cl H—F 136 n-C3H7-CH3 85 C6H5-Cl H—Cl 103 i-C3H7-CH3 84 C6H5CH2-Cl H—Br 88 t-C4H9-CH3 80 CH3-Br H—I 71 H2C=CH-CH3 92 C2H5- Br F—F 38 H2C=CHCH2-CH3 72 n-C3H7-Br CI—CI 58 C6H5-CH3 93 i-C3H7- Br Br—Br 46 C6H5CH2-CH3 70 t-C4H9- Br I—I 36 CH3-F 109 H2C=CH- Br CH3-F 108 C2H5-F 107 H2C=CHCH2-Br CH3-Cl 84 n-C3H7-F 107 C6H5- Br CH3-Br 70 i-C3H7-F 106 C6H5CH2- Br CH3-I 56 t-C4H9-F 108 CH3-I CH3—H 104 H2C=CH-F C2H5- I C2H5—H 98 H2C=CHCH2-F n-C3H7-I n-C3H7—H 98 C6H5- F 125 i-C3H7- I i-C3H7—H 95 C6H5CH2-F t-C4H9- I i-C4H9—H 91 CH3-Cl 84 H2C=CH-I H2C=CH—H 104 C2H5-Cl 81 H2C=CHCH2-I H2C=CHCH2-H 88 n-C3H7-Cl 82 C6H5- I C6H5-H 112 i-C3H7-Cl 81 C6H5CH2- I C6H5CH2-H 85 t-C4H9-Cl 79 CH3-CH3 88 H2C=CH-Cl 84 60 86 68 70 69 69 68 63 47 72 51 56 53 53 53 50 42 64 47 1 pKa of Acids H2SO4 HI HBr HCl H3O+ H-N+N H-O+R2 H-S+R2 -12 -9 -8 -7 -1.7 <0 <0 <0 CH3SO3-H H3PO4 HF CH2(NO2)2 H2S HCN NH4+ C6H5OH O O 4.8 CH3 CH3 RNH3+ CH2(CN)2 H2O Alcohols R-CCH CH3CN NH3 to R2NH alkanes RH O C C OH <0 2.2 3.2 3.6 7.0 9.1 9.2 9.9 O C CH2 10 11.2 15.7 16-19 25 25 35-36 40-60 CH3 11 O C CH3 CH3 20 O 25 C CH3 O CH3 Supplies: Common Solvents of all kinds: non-polar; hydroxylic; dipolar, aprotic Inorganic Reagents, including SOCl2, CO2, Mg, Na, K, Li, Al, NaN3, PX3, CrO3, ZnCl2, D2O, AgBr, AgNO3, RCN, NaSH, NaOH, NH3, H2SO4, HX (Cl, Br, I), NaSCN, NaH O Non-cyclic Organics: one thru five carbon alcohols, H-CC-H, CH3CO2H, CH2 CH2 , CH3SO2Cl, CH2=0, (C2H5)3N, (CH3)2SO4, "PCC", CH2=CH-CH2-OH, Cyclic Organics: Cyclopropanol to Cycloheptanol, C6H5-SO2Cl, pyridine, p-CH3C6H4SO2Cl or O CH3 S Cl O CH2 OH (for "tosylate" prep.), Conformational Energies for Monosubstituted Cyclohexanes Group: -ΔG° (axial – equatorial) Kcal mol-1 (25°C) F 0.25 Cl 0.5 Br 0.5 I 0.45 OH (in water) 1.0 CH3 1.7 CH2CH3 1.8 CCH 0.41 CH(CH3)3 2.1 C(CH3)3 5-6 C6H5 3.1 OC2H5 0.9 2 1. (07) Mandrake berries, recognized by Hippocrates (400 B.C.) for their sedative power, has a long association with murder and magic as well. Its active ingredient is the alkaloid (I). Circle and name the functional groups present in (I). N CH3 O O OH C O O 2. (10) Consider (I): a) What are the hybridizations of each of the oxygen atoms in this portion of (I) (give a symbol and an arrow for each answer)? O O H O (b) Where in (I) is there significant ∡ strain? Give a "rough” estimate" of its severity. (c)What is the name of the shape of the 5-Membered ring in (I). (Can you see it?) 3 3. (21) A recent paper (J. Am. Chem. Soc., III, 3071 (1989)) described some work on the preparation and properties of (II), F2C3=C2=C1=O (II) a) What are the hybridation of atomic orbitals, if any, at F ___________, C3_________, C2 ____________, C1 ____________ b) What are the following bond ∡ for the indicated sets of three atoms: ∡ F, C3, F = ________ ∡ C2, C1 0 = __________ c) Excluding the F atoms, what is the overall shape of the of molecule (II)? d) Sketch a lengthwise cross-section of the bond orbital found between an F atom and the C3 atom, using LCAO or MO notation. e) Using the LCAO or MO representing the nuclei element symbols and representing (sigma) bonds with lines (-----), wedges ( ) and/or dashed lines ( ), as 3-dimensions dictate, sketch the entire (pi) bond, (or equivalently, the 2p orbitals) are given space-filling characters and are orientated in accordance with spatial array that 2p orbitals have about a given nucleus. 4 EXTRA CREDIT (04) The double bond in ethylene, CH2=CH2, is 1.34 Å in length; however, in (II), the double bond between C2 and C3 in (II) is 1.30 Å. Give a reason why there has been a shortening in the double bond of C2 and C3. (Hint: Consider hybridization at the carbons and resulting properties.) 4. (11) On the planar form, IIIA, write the structural formula of cis-l-t-butyl-2-methoxycyclopentane. (See (b) 1st before adding substitutes. to (IIIA)) (IIIA) (IIIB) b) Add the two ring substituent’s of (IIIA) cis to another possible ring conformation, (IIIB). Then, comment on the relative degrees of various "strains" found in (IIIA) and (IIIB): (IIIA) Verses (IIIB) Angle Strain Torsional Strain Steric Strain 5 5. (19) Draw the structural formula of each of the following: a) Cyclooctyl vinyl acetylene b) 8-amino-5-chloro-trans, trans, cis-2,4,7-decatriene c) The structure in (b) has what E/Z designation? d) The structure in (b) is one of how many geometric isomers of the same name (excluding stereochemistry. Designation.)? 6. (16) a) Rank the basiscity of the following Lowry-Bronsted bases, in order from the most basic to the least basic. O NH3, CH3ONa, H2O, CH3C O- 6 b) Calculate the Keq and give the general position of the equilibrium: F F F F O F + OH F CH3 F O O O F + OH CH3 F F c) Whatever your answer in (b), "to the left" or "to the right", propose an alternate base in place of CH3-CO2- that will shift the equilibrium's position in favor of the "other side" and compared to the result in (b). Show a calculation. d) Which is more acidic, (IV) (above) or C6H5OH (phenol). acidity between (IV) and phenol prevails. Suggest a reason why this order of 7. (09) Name Br a) OH b) 7 (c) In (a), the alkyl bromide is a 1, 2, 3 (circle one) functional group. 8. (14) Draw the dot formula (Lewis Dot) for N2O22- ion, in which the atoms are bonded in a non-cyclic array, as follows: O to N to N to O. Caution, Count the # of valence electrons carefully! Caution: 2 answers are acceptable. b) For the dot formula in (a), calculate the formal charge at each atom. Show work! c) Draw another resonance structure of the N2O22- ion 9. (13) Consider HOCH2CH2OH, ethylene glycol (EG). a) Draw the anti and gauche staggered conformation (referring to 2 OH) and the most stable eclipsed conformation for EG. Use either Newman projection or flying-wedge style structural formulas. Draw carefully. b) What is the dihedral ∡ of the 2-OH groups in the 1st staggered conformation above, and in the eclipsed conformation? c) Usually the anti-staggered conformation of a 1, 2-disubstituted ethane is more stable. However, here the gauche staggered conformation is more stable. Suggest a reason for the gauche conformation being more stable; include an approximate sketch in your answer. Hint: How might non-H substituents interact? 10. (5) Consider the data: CH3CH2CH2-O-CH2CH2CH3 CH3CH2CH2CH2CH2CH2-OH Boiling pts °C 91 156 Solubility in H2O in g/100mL H2O 0.6 0.6 8 Account for the similar solubility in H2O yet widely different boiling pts. for the two isomers. 9