The 4 major spheres
advertisement

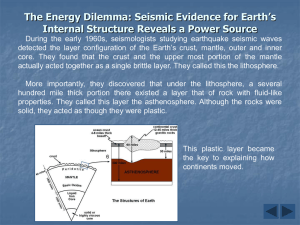
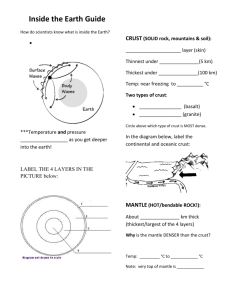
Chapter 1; Lesson 1 The Earth System [pages 2-9] System: A group of parts that work together as a whole. The Earth system is all of the Earth’s parts working together to create our planet. Energy: Part of the Earth system. Energy is the ability to do work. The 4 major spheres: hydrosphere, geosphere, atmosphere, biosphere Hydrosphere: This sphere contains all of the Earth’s water. Oceans, streams, rivers, lakes and water vapor are all examples of the hydrosphere. Geosphere: This is the Earth’s sphere that contains all of the Earth’s rock and metals. The geosphere goes all the way to the center of the Earth. Atmosphere: This is the sphere of gases that surround the Earth. The atmosphere contains such gases as oxygen, nitrogen, water vapor and carbon dioxide. Biosphere: All of the Life on Earth. The biosphere overlaps all of the other spheres. It is in the atmosphere, hydrosphere, and geosphere because life is found in all of these spheres. Constructive forces: Forces that build landforms. These include tectonic plate movement that builds mountains. Earthquakes result from plate movement so they are constructive forces. Destructive Forces: These are forces that tear down landforms. Erosion from wind and water is a destructive force. IMPORTANT FACTS: 1. Oxygen is the most abundant element on Earth. Oxygen combines with other elements, especially silicon, to form rocks. Silicon is the second most abundant element. 2. Nitrogen is the most abundant gas in the atmosphere. [78%] 3. Oxygen is the gas in the atmosphere that animals need to breathe. [21%] 4. Carbon dioxide is a greenhouse gas. It holds heat in so it can’t escape into space. [.04%] Chapter 1; Lesson 2 Earth’s Interior [pages 10-17] Two ways scientists study the inside of the Earth: 1] indirect evidence [seismic waves from Earthquakes] 2] direct evidence [rock samples from volcanoes] Scientists study how seismic waves travel through the Earth to determine how the Earth is made. The speed they travel and the paths they take give scientists clues. The 4 main layers of the Earth: 1] inner core 2] outer core 3] mantle 4] crust 1] Inner core: highest pressure and highest heat. It is a solid because the pressure is so great that liquids cannot form. The inner core is in the center of the Earth. It is made of iron and nickel and iron. 2] Outer Core: high pressure but not as high as inner core. Very hot, but not as hot as the inner core. It is a liquid because rock melts at high temperatures but the pressure is not great enough to keep it from becoming solid. It is made of the metals iron and nickel. 3] Mantle: The mantle is a solid, but it is hot and has some movement. There are pockets of molten rock in the mantle, but it is mostly solid. The mantle is the layer under the crust. There is a lot of pressure, but not as much as the inner or outer core. 4] The Crust: The outside most layer of the Earth. It is cooler than the other layers. All of life is found on or in the crust. [and the atmosphere] The crust is a solid. A] Continental Crust: The crust that is under the landmasses or continents. The continental crust is the thickest kind of crust. It is made mostly of granite. B] Oceanic Crust: The crust that is under the oceans. It is thinner than the continental crust. It is made mostly of basalt. Lithosphere: The uppermost part of the mantle and the lower part of the crust. This layer is hard and rigid. The lithosphere is about 40 miles thick. Asthenosphere: The layer beneath the lithosphere. It is hotter and under more pressure. It can bend like a spoon without breaking. Basalt: The rock that makes up the oceanic crust. Basalt is dark and fine grained. It is made of mostly oxygen and silicon. Granite: The rock that makes up the continental crust. Granite is light colored and coarse grained. It is made of mostly oxygen and silicon. INTERESTING FACTS ABOUT EARTH’S COMPOSITION: 1] The Earth’s crust is made of 46% oxygen 2] The Earth’s crust is made of 28% silicon 3] The Earth’s crust is made of 8% aluminum 4] The Earth’s crust is made of 5% iron 5] The Earth’s crust is made of 4% calcium Chapter 1; Lesson 3 Convection and the Mantle [pages 18-21] There are 3 ways that heat can be transferred. 1] Radiation: Transfer of heat through rays like light. 2] Conduction: Heat that transfers through materials that are touching. 3] Convection: Heat transfer by the movement of a fluid. [air or liquid] Convection Currents: Currents that move in a fluid that transfers heat. Hotter materials move upward because they are less dense. When they lose heat and get cooler they get more dense and sink to the bottom. Then they get hot and move upward again. This cycle continues over and over moving the material to the top and down to the bottom again. Density: How heavy something is compared to its volume. Density is always written as a ratio between volume and weight [or mass] Example: Density of Carbon is 2.3g per cm3 The density of lead is 11.3g per cm3 This means that one cubic centimeter of carbon weighs 2.3 grams, but the same size piece of lead weighs 11.3 grams which is more than 4 times as much. Lead is much more dense than carbon. Heat from the core and the mantle cause convection currents in the mantle. This is what drives plate tectonics. Plumes of mantle rock rise and fall in the mantle like lava in a lava lamp. Convection currents occur both in the outer core and in the mantle. The convection currents in the outer core cause the Earth’s magnetism. Chemistry Unit: Chemistry: The study of how matter interacts and how elements and compounds combine with other elements and compounds to make new materials. Atom: the smallest particle that has all the properties of an element. Element: A material that is made of only one type of atom. Compound: A material that is made of more than one type of atom. [The atoms are formed into molecules] Nucleus: The center part of the atom. The nucleus is made up of protons and neutrons, usually in equal numbers. Proton: A positively charged particle in the nucleus of an atom Neutron: A neutral particle in the nucleus of an atom. This means that neutrons do not have an electrical charge. Electron: Negatively charged particles that circle around the atom. Electrons are much smaller than protons and neutrons. They are arranged in shells. Valence Electrons: The electrons in the outermost shell of the atom. Valence electrons are the only electrons to interact with electrons in other atoms. They combine with other electrons to form compounds. Periodic Table of Elements: A table that shows the basic characteristics of all of the elements. They are arranged into different categories, and from least number of protons to most protons. Nobel Gases: These are gasses that do not combine with other elements, so they are not found in compounds. Atomic Number: The number of protons in the nucleus of an atom for each element. For example, oxygen is atomic number 8 because there are 8 protons in the nucleus.