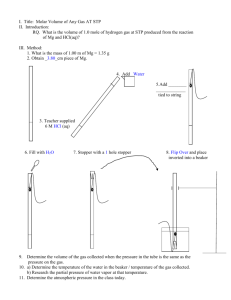
Power Point 11: Chemical Kinetics (Gas Laws)
advertisement

Power Point 11: Chemical Kinetics (Gas Laws) Drill: Calculate the mass of (NH4)2SO4 in mg produced when 1.12 mL NH3 gas reacts is excess H2SO4 in the following rxn: NH3 + H2SO4 (NH4)2SO4 Kinetic Theory: All matter is made up of tiny particles • The particles are in constant motion • All collisions are elastic Pressure: • Force per unit area • Caused by collisions against a surface • Gas measured in pressure Units of Pressure: • Pa: kilopascal (Std Unit) • Pascal: newton/sq. meter • Atmosphere (Atm): • mm Hg: Standard Pressure: • 101.3 kPa (to be changed) • 1.00 Atm • 760 mm Hg or Torrs • 30.0 inches Hg • 1013 millibars Gas Laws: • Boyle’s Law • Charles’ Law • Gay Lussac’s Law • Dalton’s Law • Graham’s Law Boyles Law: The pressure & volume of a gas at constant temperature are inversely proportioned P1V1 = P2V2 = K Charles’ Law: The volume and temperature of a gas at constant pressure are directly proportioned V1/T1 = V2/T2 = K Gay Lussac’s Law: The pressure and temp. of a gas at constant volume are directly proportioned P1/T1 = P2/T2 = K Combined Gas Law: P1V1 /T1 = P2V2/T2 Common Sense: The volume of a gas is directly proportioned to its number of moles V1/n1 = V2/n2 = K New Combination: P1V1/n1T1 = P2V2/n2T2 = K PV = nRT Ideal Gas Law Formula: Dalton’s Law: The total pressure og a gas = the sum of the partial pressures of each portion PT = P1 + P2 + etc Graham’s Law: The velocities of particles are inversely proportioned to the square root of their masses v1/v2 = √M2/M1 Drill: Calculate the new volume of 5.0 L of gas when its pressure is doubled and its temperature is tripled: Problems: Using the Gas Laws: Calculate the volume of a gas at STP when it occupied 150.0 mL at 177oC under 303.9 kPa pressure: Calculate the number of moles of gas occupying 831 mL under 250 kPa at 227oC. Calculate the volume of 3.0 moles of gas at -23oC under 83.1 kPa pressure. Calculate the ratio of the velocities of He gas to HCl gas: Calculate the mass of CO2 occupying 83.1 L under 25 GPa at 227oC Calculate the volume of a gas at STP when it occupies 80.0 mL at 273oC under 303.9 kPa pressure: Drill: Calculate the volume in mL of 4.0 g bromine gas at 127oC under 83.1 kPa pressure. Problems: Calculate the number of molecules of NH3 formed when 4.0 mg of H2 react with 0.112 mL of N2 gas at STP. Drill: Calculate the mass of Al2(SO4)3 formed when 13.3 g of AlCl3 reacts with excess K2SO4 Problems: Calculate the molecular mass of 5.0 g of gas occupying 831L under 250 MPa at 227oC Calculate the density of carbon dioxide at 27oC under 83.1 kPa pressure Integrated Formulas: PV = nRT & D = m/V & M = m/n If , M = m/n then , n = m/M, thus m/M can be substituted for n into PV = nRT This yields: PV = mRT/M or M = mRT/ PV Look at: D = m/V & M = mRT/ PV , then substitute D for m/V You get: M = DRT/ P or D = MP/RT More Problems: The total pressure of a system is 120.0 kPa. The partial pressure of gas A is 112.0 kPa. Determine the pressure of gas B Calculate the ratio of the velocities of He gas to HCl gas:. Calculate the mass of 831 mL of CO2 at 27oC under 150 kPa pressure: Drill: Calculate the volume of 4.0 moles of gas under 83.1 kPa pressure at 127oC Problems: Calculate the volume of a gas at STP when it occupies 80.0 mL at 127oC under 303.9 kPa pressure: Calculate the volume of 4.0 moles of gas under 83.1 kPa pressure at 127oC Calculate the molecular mass of 50 g of gas occupying 831mL under 250 MPa at 227oC The total pressure of a system is 120.0 kPa. The partial pressure of gas A is 112.0 kPa. Determine the pressure of gas B The total pressure of a system is 150.0 kPa. The system contains 50 % A, 30 % B, & 20 % C. Determine the pressure of each gas. Calculate the mass of CO2 occupying 83.1 mL under 25 MPa at 477oC Calculate the density of carbon dioxide at 27oC under 83.1 kPa pressure Calculate the velocity HBr when the velocity Be is 270 m/s: • Convert to Unit Problems: Calculate the volume in mL of oxygen gas at STP required to burn 50.0 mg C5H8O2: Calculate the mass of PbBr2 was obtained when 6.62 g of Pb(NO3)2 was added to 11.9 g KBr. Calculate the final volume that 3.0 L of gas will obtain when the absolute temperature is tripled & the pressure is halved. Calculate the mass of CO occupying 831 kL at 227oC under 2.50 Mpa pressure. Calculate the volume of H2 formed at 27oC under 150 kPa when 6.8 mg NH3 decomposes making N2 & H2.