8th Grade Science Chemical Reactions Chemical Reactions Extra
advertisement

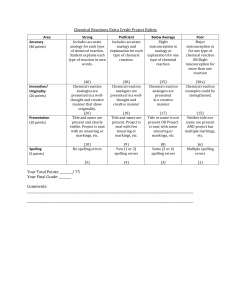
8th Grade Science Chemical Reactions Chemical Reactions Extra Credit Project This project is NOT MANDATORY. The purpose of this project is for the students to expand their knowledge of the different types of chemical reactions. There are three ways to classify chemical reactions: synthesis, decomposition, and replacement. During a synthesis reaction, multiple simple substances combine to form a more complex compound. A complex compound breaks down into two or more simpler substances in decomposition. During a replacement reaction, elements replace another element in another compound; in a single replacement reaction, one element changes its position whereas in a double replacement, two elements switch positions in two different compounds. For this project, each student will use illustration equations to represent each of the four types of chemical reactions and provide a brief explanation as to how their example shows the particular type of chemical reaction. For example, to represent a synthesis chemical equation, you could add a cat and a dog together to equal the well-known Nickelodeon cartoon, Catdog. The illustration equation for this an example could look like the following: A + B → C (AB) Cat + Dog → Catdog Catdog + → The final project is due on Monday, November 2nd . No projects will be accepted after this date! Projects must be completed on a large, white piece of construction paper provided by your teacher. This project will be counted as a test grade and will be broken up into the following categories: Accuracy: Synthesis Equation Decomposition Equation Replacement Equation (Single and Double) Innovation/Originality: Presentation (Title, Name, & Neatness): Spelling 40 points Total Points: 75 points 10 points 10 points 20 points 20 points 10 points 5 points 8th Grade Science Chemical Reactions Name: ______________________________________ Class: ___________ Date: ________________________ Chemical Reactions Extra Credit Project Rubric Area Accuracy (40 points) Innovation/ Originality (20 points) Presentation (10 points) Spelling (5 points) Strong Includes accurate analogy for each type of chemical reaction. Student explains each type of reaction in own words. Proficient Includes accurate analogy and explanation for each type of chemical reaction. Below Average Slight misconception in analogy or explanation for one type of chemical reaction. Poor Major misconception in for one type of chemical reaction OR Slight misconception for more than one reaction (40) Chemical reaction analogies are presented in a wellthought and creative manner that show originality. (20) Title and name are present and clearly visible. Project is neat with no smearing or markings, etc. (38) Chemical reaction analogies are presented in a wellthought and creative manner (35) Chemical reaction analogies are presented in a creative manner (30≤) Chemical reaction examples could be strengthened. (18) Title and name are present. Project is neat with few smearing or markings, etc. (17) Title or name is not present OR Project is neat with some smearing or markings, etc. (15) Neither title nor name are present AND project has multiple markings, etc. (10) No spelling errors (9) Few (1 or 2) spelling errors (8) Some (3 or 4) spelling errors (6) Multiple spelling errors (5) (4) (3) (1) Your Total Points: ________/ 75 Your Final Grade: ________ Comments: ____________________________________________________________________________________________________________ ____________________________________________________________________________________________________________ ____________________________________________________________________________________________________________