here - Angioslide
advertisement

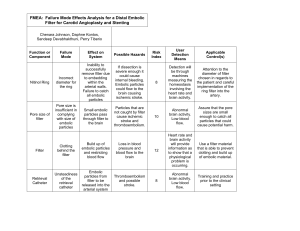
Angioslide Announces the European Launch of New 300 mm Long Device Allowing Treatment of Entire Superficial Femoral Artery (SFA) with One Device The PROTEUS™ Device Combines Balloon Angioplasty and Embolic Capture of Particles Caesarea, Israel, September 8, 2011. Angioslide Ltd. announces the first procedures with its new 5x300mm PROTEUS device for treating the Superficial Femoral Artery (SFA). PROTEUS technology combines a Percutaneous Transluminal Angioplasty (PTA) balloon and embolic capture of particles in one device. The new 5x300 mm size joins Angioslide's current dedicated product line solutions for the lower limbs. Initial treatments using the new PROTEUS device were conducted in leading world centers, Parkkrankenhaus Leipzig and Herzzentrum Bad Krozingen, located in Germany. The PROTEUS 5X300 mm makes it possible to treat lesions up to 300 mm with one device, while providing an immediate solution for embolic capture. "Angioslide’s breakthrough technology, now available also in 300 mm length as well, is a unique solution for lesions with high level of embolic material, including Chronic Total Occlusions (CTO), in stent-restenosis, thrombus containing lesions and post atherectomy PTA " said Prof. Thomas Zeller, Head of the Angiology Department, Heart Center Bad-Krozingen. Prof. Dierk Scheinert, Head of the Department of Angiology, Park-Krankenhaus Leipzig adds "We have been using the PROTEUS product line as part of our daily practice for a few years, and are working closely with the Angioslide team. I welcome the release of the 5X300 mm. We can now use one device to treat patients with SFA diseases, which facilitates the procedural flow and makes the intervention cost-effective.” With the increase in life expectancy, diabetes prevalence, and number of high-risk patients, together with the shift toward an "endovascular-first" approach as a preferred procedure over surgical revascularization, there is a growing need for expanding the current interventional “tool box” to accommodate procedures designed specifically to treat long and diffuse lesions in a quick and effective manner. “We are committed to introducing new products to the market continuously, addressing the growing range of endovascular solutions," said Lihu Avitov, Angioslide CEO. "With patients’ needs in mind, the 5X300 mm device is the first in a series of long balloons, adding to our current offering of 20-100 mm devices. The PROTEUS product family provides additional confidence to clinicians treating their endovascular patients.” The PROTEUS 5X300 mm is currently released for the European market only, and is undergoing an evaluation regulatory process with the FDA. About Angioslide Founded in 2005, Angioslide is a privately held medical device company that developed a unique Embolic Capture Angioplasty solution, PROTEUS™, which provides a combination of PTA balloon and embolic capture. The PROTEUS™ device addresses an unmet need for an easy-to-use, efficient and cost-effective embolic particle capture solution for the peripheral vascular disease market. It is the first device of its kind to receive FDA clearance for use in lower limbs. The device has also received European CE Mark approval for lower limb use and is being marketed in selected regions in Europe. Angioslide headquarters are located in Caesarea, Israel, with regional offices in Europe and the US. For more information visit our website at www.angioslide.com Contact: Shira Doron Marketing Director Mobile: +972-54-9011134 Email: shirad@angioslide.com