Heat Mixes P3
advertisement

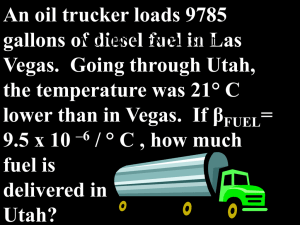
21st Century Cohort Lesson Plan Components The best instructional designs are “backwards,” beginning with the end in mind and moving through three interrelated stages. As we design instruction, we must begin with desired results (Stage One); then determine how to assess and evaluate student progress relative to those results (Stage Two); and only then design and implement instructional strategies to promote student achievement of desired results (Stage Three). Desired results must clearly specify what students are expected to know (e.g., facts, concepts, generalizations, rules, principles, laws); do (e.g., skills, procedures, processes); and understand (e.g., student behaviors reflecting the six facets of understanding) as a result of the teaching and learning process. 6 Facets of Understanding: 1. Explanation 2. Interpretation 3. Application 4. Perspective 5. Empathy 6. Self Knowledge Stage One – Begin with Desired Results: What do you want students to know, be able to do, and understand? What are your learning targets? Learning Target One – I am able to predict the final temperature when water of different temperatures are mixed together. Learning Target Two – I am able to explain the method of temperature prediction. Stage Two – Assess and Evaluate Assessment Evidence: How will you know they have accomplished the learning targets? (performance task / quizzes / observations / homework / journals / projects / writings / speeches / etc) Which strategies will provide evidence of student learning? Assessment Evidence One – Students can successfully predict the final temperature. Assessment Evidence Two – Students can write the method of temperature prediction in the laboratory write up. Stage Three – Design and Implement Learning Plan: What instructional strategies will you use to help students achieve the learning targets? Which instructional strategies will help students acquire and integrate learning? Which instructional strategies will help students practice, review and apply learning? Which strategies help students practice and improve their 21st century skills? Learning Plan: 1. Have students complete a reading guide on thermal energy. 2. Discuss the three components of thermal energy. 3. Perform a lab to predict a final temperature. 4. Predict the final temperatures and compare to the actual final temperature. (The Lab procedure follows) Heat Mixes Equipment: 3 Styrofoam cups, liter container, thermometer, cold water, hot water Discussion: If you mix a pail of cold water with a pail of hot water, the temperature of the mixture will be between the two initial temperatures. What information would you need to predict the final temperature? This lab investigates what factors are involved in changes of temperature. Your goal is to find out what happens to the final temperature when you mix equal masses of water at different temperatures. Preliminary Questions: Before actually doing this, imagine a cup of water at 60o C and a pail of water at 20o C. 1. Which do you think is hotter-the cup or the pail? 2. Which do you think has more energy? 3. Which would take longer to change its temperature by 10o C if left along? 4. If you put the same amount of red-hot iron into the cup and the pail, which one would change temperature more? Procedure: Step 1: Fill one cup ¾ full with cold water. Mark the level along the inside of the cup. Pour the cup’s water into a second cup. Mark it as you did the first one. Pour the cup’s water into a third cup, and mark it as before. Now all three cups have marks that show nearly equal measures. 5. Why don’t the marks show exactly equal measures? Step 2: Fill the first cup to the mark with hot water. Measure and record the temperature of both cups. The cold cup from step 1 and the hot cup from step 2. Temperature of cold water ___________oC Temperature of hot water ___________oC Step 3: What will be the temperature is you mix the two cups of water in the container? Predicted temperature of mixture ___________oC Step 4: Pour the two cups of water into the container, stir the mixture, measure and record its temperature. Actual temperature of mixture ___________oC Pour the mixture into the sink. 6. How did your observation compare with your prediction? If there was a difference between your prediction and your observation, what might have caused it? Step 5: Fill the cups to the marks, one with cold water and two with hot water. Measure and record their temperatures. Temperature of cold water ___________oC Temperature of hot water 1 ___________oC Temperature of hot water 2 ___________oC Step 6: Record your prediction of the temperature when all three cups of water are mixed into one container. Predicted temperature of mixture ___________oC Step 7: Pour the three cups of water into the container, stir the mixture, measure and record its temperature. Actual temperature of mixture ___________oC Pour the mixture into the sink. 7. How did your observation compare with your prediction? If there was a difference between your prediction and your observation, what might have caused it? 8. Which of the water samples (cold or hot) changed more when it became part of the mixture? Why do you think this happened? Step 8: Fill the cups to the marks, one with hot water and two with cold water. Measure and record their temperatures. Temperature of cold water 1___________oC Temperature of cold water 2 ___________oC Temperature of hot water ___________oC Step 9: Record your prediction of the temperature when all three cups of water are mixed into one container. Predicted temperature of mixture ___________oC Step 10: Pour the three cups of water into the container, stir the mixture, measure and record its temperature. Actual temperature of mixture ___________oC Pour the mixture into the sink. 9. How did your observation compare with your prediction? If there was a difference between your prediction and your observation, what might have caused it? 10. Which of the water samples (cold or hot) changed more when it became part of the mixture? Why do you think this happened?