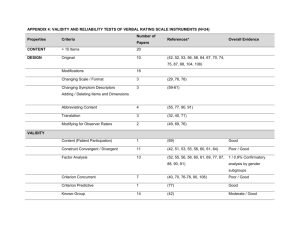
BioCOSHH Guidance - Research Complex at Harwell
advertisement

Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 1 of 13 Guidance to completing the Generic Risk Assessment for work involving Biological Materials (taken from University of Durham) NOTES: i. Current Policy – A) The following areas of work may not be undertaken without taking advice from the Biological Safety Officer (BSO) and completing this form before work starts. Work involving ‘biological materials’ or 'biological agents'. This will include the use of experimental materials which may be contaminated with a biological agent. Work involving genetic modification or work with genetically modified organisms. Work with blood or body products. B) “Prescribed Pathogens” must not be brought onto the premises in any quantity without notifying the Biological Safety Officer of the intention to do so. Any such work will require the prior approval of STFC and completion of a risk assessment form. ii. This document is intended to guide investigators through an initial risk assessment for a proposed research project involving biological materials (See definition of biological materials in Additional Notes below*). Consideration must be given to the possible risk of harm or damage to humans, animals and the environment through the proposed work with the biological materials. iii. Of particular concern are the following examples of biological materials which will require special containment facilities or licences – human or primate blood/serum, body secretions or tissues; human, cattle, sheep, deer brain tissues; live animals, any materials likely to carry human pathogens; deliberate use of named human pathogens, parasites or zoonoses; pathogens causing diseases in crop plants; diseased plant materials; genetically modified microorganisms, plants or animals. iv. These are generic guidelines so not all sections or questions may be appropriate for all types of work or experiments. Investigators may use their own risk assessment form but should ensure that all the relevant information requested below is provided in their form and should be made appropriate for your Department. If in any doubt please contact the Biological Safety Officer. v. Where appropriate brief examples are provided for each question for illustrative purposes; in most cases more information will be necessary. vi. Based on the risk assessment a Containment Level for the work will need to be assigned: CL1, 2 or 3, (3 being the highest level, required for work on hazardous pathogens, see below) and, as appropriate, the work will need to be carried out in a suitably equipped laboratory facility with codes of practice for the import, handling, storage, disposal and emergency procedures involving the specified biological materials will be required. The staff and students involved will need to be trained in all procedures. All projects assigned a level of CL2 or above require to be approved by the STFC Biological Safety Committee and the risk assessment should be forwarded to the BSO for this purpose. vii. Copies of the final completed risk assessment form should be retained by the investigator and the departmental biological safety coordinator. viii. Depending on the nature of the proposed research a further risk assessment and approval from the safety Committee (BGMSC) or other regulatory authorities (ACGM, DEFRA, Home Office) may ALSO be necessary. Application for a licence may be necessary. Document1 Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 2 of 13 ix. Normally purified proteins (serum albumin), enzymes (nucleases), nucleic acids (E.coli DNA), blood products (sera) or other specified extracts (tryptone) obtained from reputable commercial suppliers should not require risk assessments, however the safety data sheets accompanying these products should be examined for any statements referring to hazards, pathogen screening etc. x. The BSO can advise on most aspects of work with biological materials and on the completion of this form. 1. Proposer Name: Department: Contact number(s): E-mail: Staff / Students involved in the project: Explanatory Notes Provide contact details for the person responsible for the work – the principal investigator/grant holder/ team leader and list all co-investigators, technicians, postgraduate students or anyone else involved directly in the work. Keep this list up-to-date by adding new staff and students when they start work. All personnel associated with the work should read this risk assessment and be aware of its implications. Appropriate guidance, supervision and training must be provided. 2. Description of the proposed work Provide a brief description of the aims and objectives of the work example: “This project is designed to investigate responses of plants to insect pests. Experiments will involve exposing leaves of test plants to different types of insect pests and measuring the amount of leaf damage over a short time. Transgenic plants containing anti-insect compounds will be produced for these tests. The experiments will be carried out in CL1 and CL2 GM labs, and a GM glasshouse in Biological Sciences. The work will involve genetically modified plants and micro-organisms.” Document1 Say what you want to do. This section is to provide an overview of what the research project is about and what methods and facilities will be used. Try to avoid technical language / jargon so that the description can be understood by a non-expert. Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 3 of 13 3. What is the nature of the biological material? a. Describe all the biological materials likely to be used in the work examples: microorganisms (E.coli - lab strains, yeast – lab strains) for production of expressed proteins. Small – medium scale cultures ~ 1ml up to 10L sputum samples taken from human (student) volunteers ~1ml samples from 150 subjects potato tuber samples of several different varieties; several hundred samples of up to 500g tubers. Staphylococcus aureus MRSA strains (a category 2 pathogen) small scale cultures and samples used to prepare slides for microscopy. Describe what biological materials will be used – will the work involve live animals (including humans), tissues, faecal, blood, soil or water samples, micro-organisms, viruses, plant seeds? Provide as much information as you have available. How much of these materials will be handled? Numbers of samples; volumes or weight of samples/cultures. Note: all experimentation with humans and human materials, primates or primate-derived materials will require permission from an Ethics Committee before work can proceed. 3. (Continued) b. Briefly describe how and where the materials will be obtained from and where these will be used. examples: materials will be supplied from a commercial company sent through the post in secure packaging. obtained from a reputable collaborating laboratory/hospital (specify) ~50 hair samples will be collected from wild monkeys from a colony in West Africa and transported by the investigators to the UK. disease-free genetically modified plant materials will be grown from seed in specific glasshouses in the botanic gardens microorganisms will be cultured in a containment laboratory in the Department / in a collaborating University human blood samples screened for major blood-borne viruses (HIV and hepatitis A, B, D) healthy rabbits (6), checked by the Named Veterinary Surgeon and unlikely to be carrying any zoonoses Where and how will you obtain the materials? Where and when will the samples be examined – e.g. used immediately ‘in the field’ or brought back to a facility for storage, assay or examination? Will the samples be ‘screened’ to rule out the presence of defined harmful biological agents? c. Describe how the materials will be treated and stored examples: Soil micro organisms cultured and DNA extracted using phenol (DNA) Ecoli clones stored on agar slopes at 4oC or in glycerol for low-temperature storage rabbit blood sampled directly into a formalin fixative Document1 The way in which biological materials are treated and stored may increase or decrease the hazard. Specify any pre-treatment of the materials - chemical extraction, culturing, disinfection, freezing, fixation, autoclaving, heat treatment or other processing which could affect agents (inactivate or amplify) Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 4 of 13 grouse faecal samples dried in an oven at 100oC for 24h clarified sewage samples treated with hypochlorite for 24h fox carcass tissue samples cultured in nutrient broth overnight present. Codes of practice (CoP) for treatment of samples must be prepared where such processing is used as a means of removing pathogens / agents and evidence that this is a validated method (ie evidence that it kills the agent) attached to the CoP. 4. What biological agents might be present in these biological materials? a. Name each biological agent which may pose a risk of harm to human health, animals or the environment examples: Unscreened human blood may carry the viruses causing hepatitis, AIDs Bovine brain tissue may carry infective prions causing CJD in humans Deer carry ticks which may transmit Lyme disease Human sputum samples may carry M.tuberculosis (TB) Staphylococcus aureus (MRSA) cultures from nasal swabs Agrobacterium tumefaciens is a soil-borne pathogen of dicotyledonous plants animal dander or urine may cause allergies Most biological materials unless pre-treated will have associated biological agents which may pose a risk to human health. List all known or possible agents which will be used or may be present in the biological materials. Include viruses, prions, bacteria, fungi or parasites (such as worms or potozoa). Include experimentation with defined microorganisms and provide details of the designations, genotype etc. While plant pathogens pose no direct risk to human / animal health they may pose an environmental risk (crops). See section (section 4f). A Defra plant health licence may be required. If the biological material also contains a toxin or other biologically active chemical which may pose a risk list it here and proceed to the toxin / chemical risk section (section 5) 4. (Continued) b. Indicate the hazard group that the agent has been assigned to (see guidelines). examples: ( taken from ACDP information) Hepatitis is a HG2 viral pathogen – all unscreened human blood must be handled at CL2 or above Proteus is a HG2 bacterial pathogen Foot & Mouth Rhinovirus is a group 2 animal pathogen subject to additional restrictions (Defra) Paracoccidiodes brasiliensis is a HG3 fungal pathogen requiring CL3 facilities Most Eschericia coli strains are HG1. Haemorrhagic strains such as O157 are HG2 M.tuberculosis is a CL3 pathogen Identified human pathogens are assigned to a Hazard Group (HG1-4) depending on their human pathogenicity and effective measures of treatment against the disease - see additional notes below. This dictates the containment level (CL1,2 or 3) of facilities needed to work with this pathogen or material. Consult the ACDP guidance on Categorisation of Biological Agents. The combination of the hazard group and the likely incidence of the agent will determine the containment level that the work should be carried out at. c. For each named agent provide information (if available) on the likely presence and prevalence in the material examples: Blood samples will be screened for all major Document1 Provide an informed estimate of the likelihood of occurrence and incidence of each agent in Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 5 of 13 viral pathogens so the likelihood of the presence of any harmful agents is low Samples will be collected from wild birds which may have a moderate to high risk of carrying Campylobacteriosis (category 2 pathogen) Sputum samples taken from unscreened volunteers – there is some likelihood of the presence of M.tuberculosis (TB bacterium); incidence from medical evidence has been estimated to be 10% of this population Small whole blood samples taken from a wild colony of macaque monkeys in Sumatra – there is no reported incidence of simian herpes B or SIV (siminian immunodeficiency virus) in this colony or in this geographical area the material. This may depend on the original source of your material and if any screening has been carried out (1b). For further examples see Table 1 below for estimates of the incidence of named zoonoses in experimental animals. Sources of information may include web sites, literature sources or your colleagues. Collaborators who have already carried out a proper (written) risk assessment are extremely valuable sources of information. All sources of information should be quoted and attached if they support your risk assessment. d. Provide brief information (if available) on mode of transmission of the named agents, disease caused and symptoms. examples: hepatitis is spread through contact with blood and blood products or contaminated hypo needles …is spread through inhalation of aerosol droplets.. agent causes respiratory infection with symptoms similar to pneumonia…. To cause harm microorganisms and other agents must gain access to the host. What is the route of infection by each agent. What disease and symptoms are produced. Is there an effective treatment for the disease e. Provide information (if available) on the viability of the named agents/pathogen. examples: Outside of living host cells the virus is short lived and will remain viable for less than an hour Prions will survive normal autoclaving and heat treatment regimes Bacterial spores such as B.anthracis are extremely resistant to heat, dehydration and disinfectants f. Environmental risk Examples Agrobacterium is a pathogen of dicotyledonous plants which could affect crop species Phytophthora infestans is a potent pathogen of potato and other Solanaceous plants / crops Under what conditions will the agent survive, propagate or be killed. What disinfectants are effective against the agent. Consider the pretreatment of the material and treatment prior to disposal. Describe the consequences of the escape of experimental organisms into the neighbouring environment – consider plant pathogens, transgenic plants, transgenic animals, animal pathogens. 5. What other harmful substances might be present in these biological materials? a. Name any toxin or biologically active substance which might be present and may pose a hazard to human health. Indicate routes of exposure example Ricin is a potent cytotoxin (ribosomeinactivating protein) extracted from caster beans and in seed flour. Toxic effects follow ingestion, injection or inhalation of dust Document1 List all known or likely harmful chemical toxins which may be present in the biological materials – including toxins, irritants, corrosive chemicals, allergens, drugs or any other biologically active substance. Consult web sites, literature sources, Health and Safety Office, collaborators or your colleagues for lists of likely harmful substances in your biological materials. Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 6 of 13 (protein extract or flour). Seeds also contain potent allergens – skin and inhalation b. Indicate the likely harmful effects of these substances on human health. example Ricin is a cytotoxin which causes sickness, fever leading to coma and death within 48h c. For each substance provide information (if available) on its likely concentration in the material. Describe the nature of the substance and its harmful effect – what are the symptoms of exposure; is there an effective treatment or antidote?. Provide any known information on the level of harmful substances in your materials. example Ricin is a potent toxin with an LD50 (lethal dose) of 0.5mg / Kg body weight d. What level of each substance has an effect on human health. How does the substance enter the body? Provide any information on the dose-response characteristics. example Ricin is lethal at 1g/kg body weight. The amount of ricin in a single seed is potentially lethal 6. Specify risk controls for the work a. Considering the factors detailed in the previous sections suggest the level of containment required for the work (usually CL1-3). example “Culture of all the S.aureus strains will be carried out in the CL2 facility…..” b. Suggest any special precautions required for equipment or operations (codes of practice) example “Small – medium scale cultures will be grown in a 10L fermenter in the CL2 facility. Cells will be processed in a class 2 microbiological safety cabinet in the CL2 laboratory to contain aerosols” c. Provide details of training of staff / students who will be involved in the work example “All staff and students involved in this work are trained by experienced post-doctoral staff; they receive copies of RA and all relevant CoP’s d. List and attach any supporting documentation and codes of practice example “codes of practice, papers quoting prevalence of pathogen, risk assessment from collaborating institute are attached” Document1 Consider the likelihood, incidence and the hazard groups of all the possible agents in the materials plus any other relevant information, assign a suitable containment level for the work. This in turn will decide the specifications of the laboratory and the codes of practice necessary to perform the work. Containment facilities for working with specified pathogens involving their culture is defined by their hazard grouping – HG2 requires CL2 facilities. The detailed specifications for containment facilities is provided in ACDP documents “THE MANAGEMENT, DESIGN AND OPERATION OF MICROBIOLOGICAL CONTAINMENT LABORATORIES” and “CATEGORISATION OF PATHOGENS ACCORDING TO HAZARD AND CATEGORIES OF CONTAINMENT” Provide full justification for the assignment. Note that for all containment categories above CL1 (basic level), the BSO must be notified and permission obtained before the work can commence. Attach to the final risk assessment any documents, letters or references which support the assignment of the level of containment. Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 7 of 13 7. Does the import, production and use of the biological material require a special licence, notification or specific permission from a legislative body or a University committee? Consider the legislative and advisory bodies covering operations with the biological materials or agents / harmful substances which may be contained in the materials. example “Permission for these experiments to proceed has been granted by the Ethics Committee (03/09/04)” “This risk assessment has been passed to the BGMSC for consideration at the next meeting” “Notification of the intention to carry out this research has been prepared for submission to HSE along with this RA when approved” Signature: Department: Date: Document1 Because of the nature of the biological materials it may be necessary to obtain a licence or written permission for its import from abroad or from another lab (specific licensed pathogens; GM animals/plants; soil samples). In particular consider the possibility of pathogens or harmful substances which might be present in the experimental materials. Consult with the appropriate legislative body (Web site). Note: experimentation with humans and human materials, primates or primate-derived materials may require permission from an Ethics Committee before work can proceed. Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 8 of 13 Abbreviations ACDP CoP DBSC GM Abbreviations Advisory Committee on Dangerous Pathogens Code of Practice RA Health and Safety Executive Risk Assessment Departmental Biological Safety Coordinators Genetic Modification (Genetically Modified) GMM Genetically Modified Microorganism GMO Genetically Modified Organism (GM Plant /GM Animal) Document1 HSE BSO Biological Safety Officer Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 9 of 13 Additional Notes: A) Definition of Biological materials For the purposes of this document "Biological materials" means any biologically-derived materials or materials which, either by accident or design, contain biological agents including bacteria, viruses, micro-organisms, genetically modified organisms / microorganisms (GMOs, GMMs), prions, or any other biological agents which might pose a risk to health and safety or the environment. This includes 1) the intentional use of specified micro-organisms, viruses or other biological agents, genetically modified organisms / micro-organisms (GMO’s, GMM’s); 2) Use of materials which may incidentally contain such agents; 3) Additionally biological materials which may contain toxic or harmful chemicals; 4) Live animals. B) Biological Agents: The following items are extracted sections from the COSHH regulations relating to biological agents – numbers refer to sections: 21. The definition of a ‘biological agent’ includes: (a) micro-organisms such as bacteria, viruses, fungi, and the agents that cause transmissible spongiform encephalopathies (TSEs); (b) parasites, eg malarial parasites, amoebae and trypanosomes; and (c) the microscopic infectious forms of larger parasites, eg the microscopic ova and infectious larval forms of helminths; providing they have one or more of the harmful properties specified in the definition (cause any infection, allergy, toxicity or otherwise create a hazard to human health). Most are infectious but some agents can be harmful in otherways, for example, via the production of toxins or by inducing allergic responses. 22. Biological agents are classified into four hazard groups according to: (a) their ability to cause infection; (b) the severity of the disease that may result; (c) the risk that infection will spread to the community; and (d) the availability of vaccines and effective treatment. 23. The four groups of biological agents and their accompanying descriptions are set out in paragraph 2(2) of Schedule 3 to these Regulations. Biological agents in Groups 2 to 4 are listed in a classification list approved by HSC (referred to as the Approved list of biological agent^). The Approved List may be viewed on the Internet at http://www.hse.gov.uk/press/2004/e04078.htm (The document itself is at http://www.hse.gov.uk/pubns/misc208.pdf). The List is not exhaustive and a biological agent that does not appear on it does not automatically fall into Group 1. Even where a non-infectious biological agent does fall into Group 1, substantial control measures may still be needed for it, depending on its other harmful properties. Document1 Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 10 of 13 C) Hazard groupings based on the likelihood of significant disease resulting from infection by the pathogen Hazard Group 1: Hazard Group 2: Hazard Group 3: Hazard Group 4: A biological agent unlikely to cause human disease. A biological agent that can cause human disease and and may be a hazard to employees; it is unlikely to spread to the community and there is usually effective prophylaxis or effective treatment available. A biological agent that can cause severe human disease and presents a serious hazard to employees; it may present a risk of spreading to the community, but there is usually effective prophylaxis or treatment available. A biological agent that causes severe human disease and is a serious hazard to employees; it is likely to spread to the community and there is usually no effective prophylaxis or treatment available. This category is highly specialised and restricted to research hospitals or infectious disease research institutes D) Examples of human pathogens potentially carried and spread by natural populations of animal species (Zoonoses). The table is not all-inclusive and consideration should always be given to any special factors which may affect your risk assessment. The table should be used for initial guidance only since ACDP advice may be updated at any time. For accurate info please refer to current ACDP Guidance at HSE website. Document1 Generic Risk Assessment Guidance Issue: 1 Date: November 2009 Page 11 of 13 ZOONOSIS PREVALENCE IN HOST HAZARD GROUP OF CAUSATIVE AGENT Salmonellosis Low-moderate 2+*+ Leptospirosis Low 2 Lymphocytic choriomeningitis (LCM) Low 3 Campylobacteriosis Moderate 2 Hantavirus disease (pulmonary and renal syndrome Moderate 2/3 Rat bite fever (STREPTOBACILLUS) Moderate 2 Leptospirosis Low-moderate 2 Hantavirus disease Low 2/3 Campylobacteriosis Moderate 2 Salmonellosis Moderate 2+*+ Lymphocytic choriomeningitis (LCM) Low 3 Salmonellosis Moderate 2+*+ Pasteurellosis Moderate 2 GUINEA PIGS Salmonellosis Moderate 2+*+ FERRETS Salmonellosis Low-moderate 2+*+ Tuberculosis Low 2/3 Salmonellosis Moderate 2+*+ Toxoplasmosis Moderate-high 2 Cat scratch fever (BARTONELLA HENSELAE) Low 2 Cowpox infection Low 2 Tuberculosis Low 3 Chlamydiosis Low 2 HOST SPECIES MICE/RATS MONILIFORMIS AND SPIRILLUM MINUS) VOLES/SHREWS HAMSTERS RABBITS CATS Document1 Generic Risk Assessment Guidance DOGS SHEEP/GOATS PIGS CATTLE Document1 Issue: 1 Date: November 2009 Page 12 of 13 Campylobacteriosis Moderate-high 2 Leptospirosis Low 2 Salmonellosis Low 2+*+ Toxocariasis Moderate 2 Pasteurellosis Moderate 2 Cryptosporidiosis Moderate 2 Q fever Moderate 3 Orf virus infection Moderate 2 Salmonellosis Low-moderate 2+*+ Louping ill Low 3 Toxoplasmosis Moderate 2 Ovine chlamydiosis (enzootic abortion) Moderate 2 Erysipelas Low 2 E. COLI 0157 infection Low-moderate 2 STREPTOCOCCUS SUIS infection Moderate 2 Salmonellosis Moderate 2+*+ Campylobacteriosis Moderate 2 Erysipelas Moderate 2 Cryptosporidiosis Moderate 2 Leptospirosis Moderate-high 2 Q fever Moderate 3 Ringworm (eg TRICHOPHYTON spp) Moderate 2 Salmonellosis Moderate 2+*+ Tuberculosis Low 3 Generic Risk Assessment Guidance BIRDS NON-HUMAN PRIMATES REPTILES (eg lizards) (eg terrapins) Aquatic vertebrates Invertebrates(eg ticks) Aquatic invertebrates(eg shellfish) Issue: 1 Date: November 2009 Page 13 of 13 E. COLI O157 infection Moderate 2 Campylobacteriosis Moderate-high 2 Chlamydiosis Moderate 3 Salmonellosis Low 2+*+ Newcastle disease Low 2 MYCOBACTERIUM AVIUM infection Moderate 3 Marburg virus Rare 4 Ebola virus Rare 4 HERPESVIRUS SIMIAE (Simian herpes B virus) Moderate-high 3 Tuberculosis Low 3 Salmonellosis Moderate 2+*+ Shigellosis Moderate 2/3 Campylobacteriosis Moderate 2 Helminth infection Low 2 Other herpes viral infections Moderate 2 Salmonellosis Moderate-high 2+*+ Mycobacterial infection Low 2/3 Ehrlichiosis Low 3 Lyme disease Moderate 2 Louping ill Moderate 3 Various viral gastrointestinal diseases - organisms which filter feed concentrate viruses, eg Norwalk Moderate 2 hepatitis A +*+ SALMONELLA TYPHI AND S. PARATYPHI are in Hazard Group 3 Document1